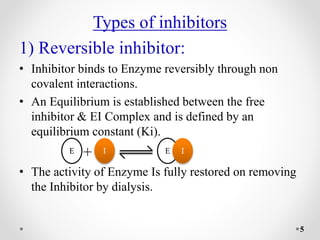
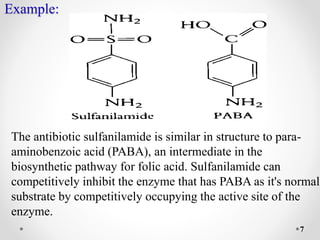
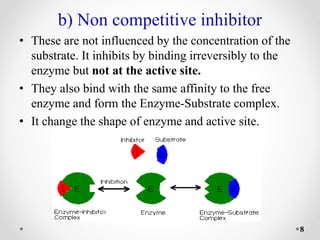
Reversible and irreversible enzyme inhibitors can be classified based on their binding interactions with enzymes. Reversible inhibitors form non-covalent complexes with enzymes and their activity can be restored upon removal of the inhibitor. Irreversible inhibitors form covalent bonds and permanently inactivate the enzyme. Reversible inhibitors include competitive inhibitors, which bind the active site, non-competitive inhibitors, which bind elsewhere and alter the enzyme's shape, and uncompetitive inhibitors, which only bind the enzyme-substrate complex. Irreversible inhibitors are either active site directed, covalently binding the active site, or suicide inhibitors, which are transformed by the enzyme into a reactive molecule that inactivates it. The Michaelis-Menten equation





![c) Uncompetitive inhibitor
• Uncompetitive inhibitors do not bind to the free
enzyme. They bind only to the enzyme-substrate
complex to yield an inactive E. S. I complex.
• Uncompetitive inhibitors frequently observed in multi
substrate reaction.
• Inhibition can’t be reversed by increasing the [S]
since I doesn't compete with S for the same binding
site.
Enzyme
Enzyme
S
Enzyme
I
S
10](https://image.slidesharecdn.com/enzymeinhibitorsreversibleandirreversible-160930101318/85/Enzyme-inhibitors-reversible_and_irreversible-10-320.jpg)



![Michaelis-menten equation
The michaelis-menten equation arises from the
general equation for an enzymatic reaction.
E+S ES E+P
The michaelis menten equation is:
V˳=
Where=
V˳= velocity of the reaction
Vmax= maximal rate of the reaction
[substrate]= conc. Of the subcstrate
Km= michaelis-menten constant
Vmax [S]
km+[S]
14](https://image.slidesharecdn.com/enzymeinhibitorsreversibleandirreversible-160930101318/85/Enzyme-inhibitors-reversible_and_irreversible-14-320.jpg)

