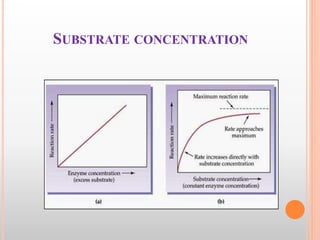
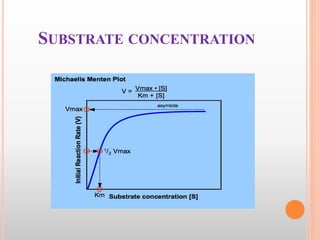
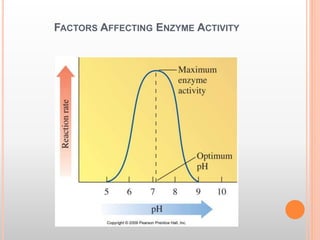
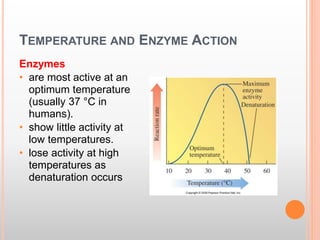
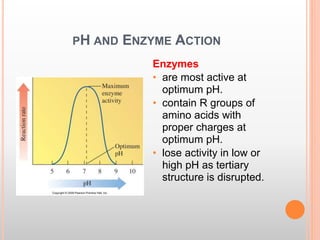
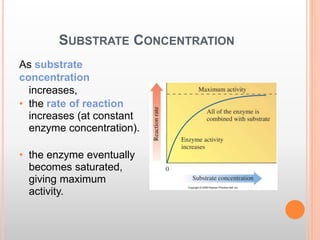
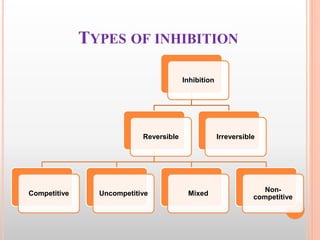
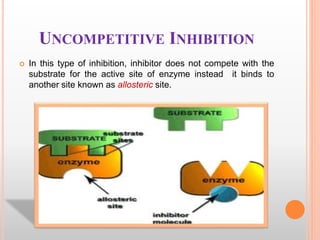
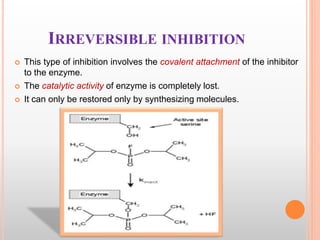
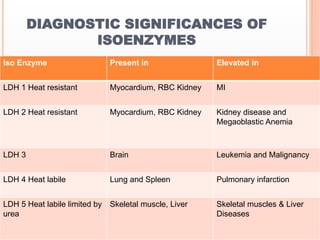
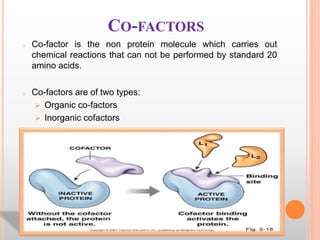
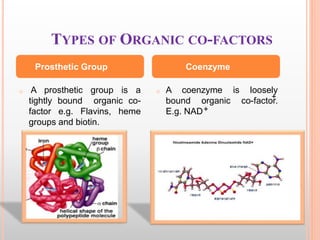
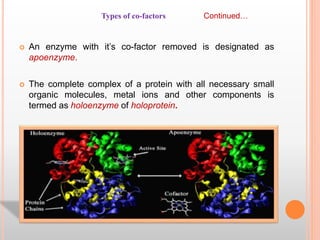
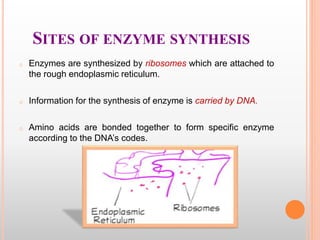
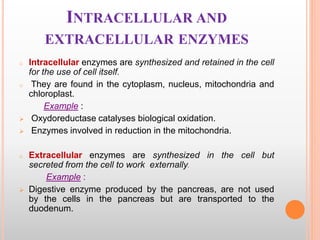
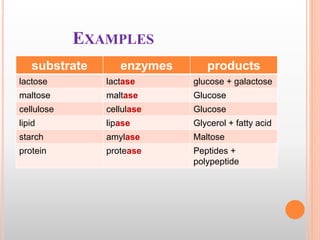
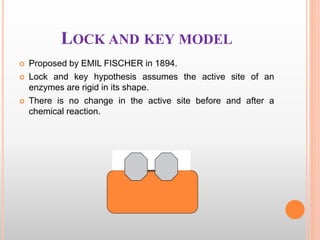
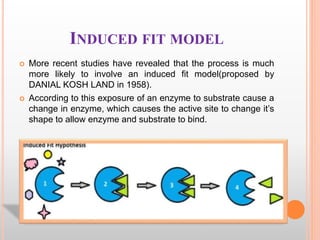
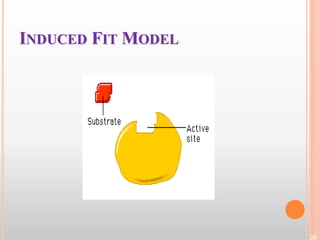
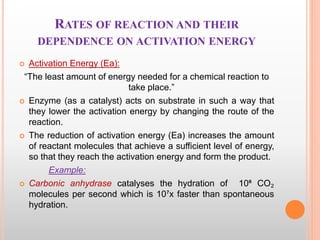
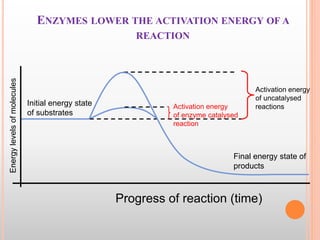
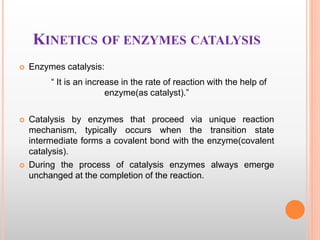
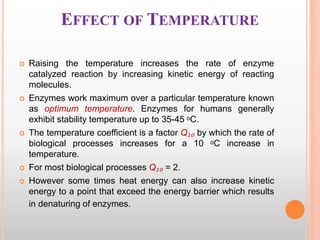
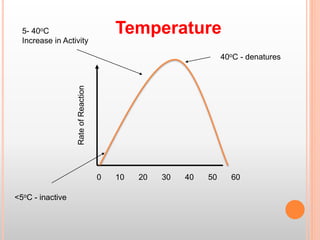
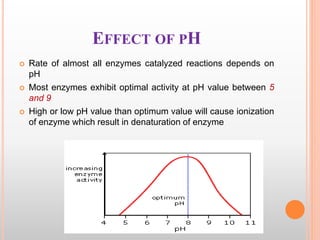
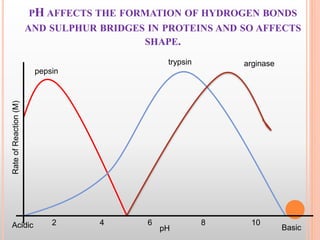
This document discusses enzymes and provides information on their chemistry, classification, mechanism of action, kinetics, inhibition, activation and specificity. It defines enzymes as biological catalysts that speed up biochemical reactions. Most enzymes are globular proteins that contain an active site for substrate binding. The document outlines different types of enzyme kinetics including effects of temperature, pH, and substrate concentration. It also describes different types of inhibition like competitive, non-competitive and irreversible inhibition. Activation of enzymes by cofactors is also summarized.









![MICHAELIS-MENTEN EQUATION
Michaelis-Menten Equation:
“It is an equation which describes how reaction velocity varies
with substrate concentration.”
Vmax [S]
Vo=
Km+[S]
Where
Vo is the initial reaction velocity.
Vmax is the maximum velocity.
Km is the Michaelis constant = (k₋₁+k₂)/k₁.
[S] is the substrate concentration.](https://image.slidesharecdn.com/enzymes-160517003157/85/Enzymes-Biochemistry-38-320.jpg)