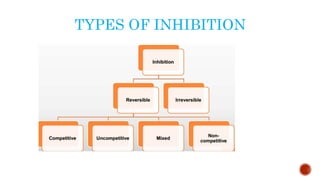
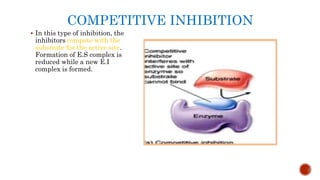
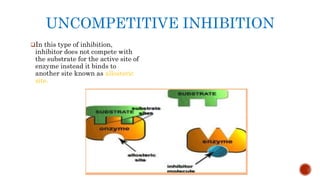
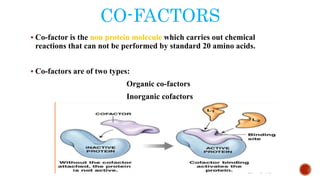
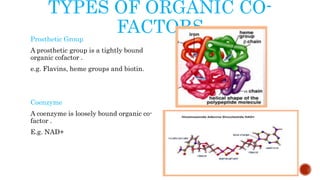
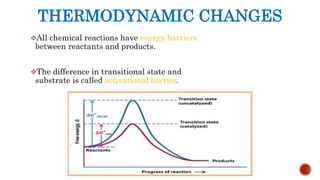
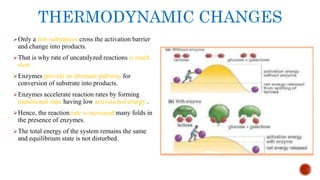
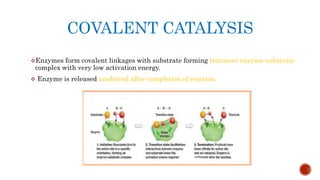
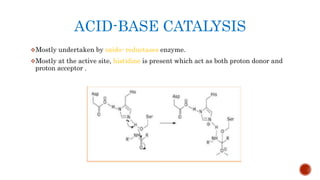
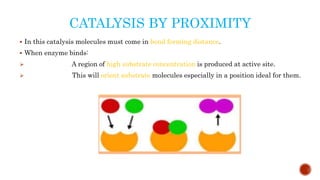
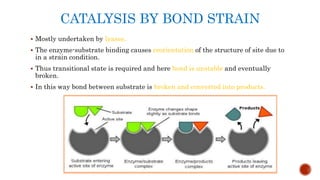
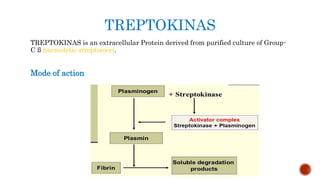
This document provides an overview of enzymes and their uses in therapy. It discusses what enzymes are, their characteristics, activation processes, inhibition types, cofactors, and mechanisms of action. It then describes applications of enzymes in the pharmaceutical industry for analytical, industrial, and therapeutic purposes. Specific examples are given for treating diseases like cancer, diabetes, and heart attacks. Assay methods for measuring enzyme activity are also outlined. Finally, fibrinolytic drugs used to break up blood clots are explained, focusing on streptokinase.