This document provides information about electron configuration and the principles that define how electrons are arranged in an atom's orbitals. It discusses the ground-state electron configuration as the most stable, lowest-energy arrangement of electrons in an atom. Three main rules that define electron configuration are described as the Aufbau principle, Pauli exclusion principle, and Hund's rule. The document also explains how electron configuration can be written using orbital diagrams or notation, and how the noble gas notation is used.




 represents the electron configuration
for helium, 1 s 2
,
[Ne]( atomic number =10) represents the electron configuration
for neon,
1 s 2
2 s 2
2 p 6
.](https://image.slidesharecdn.com/electronconfigurationlecturenotes-170316101902/85/Electron-configuration-lecture-notes-5-320.jpg)
![ Noble gas notation of sodium :
ElectronConfiguration notation of sodium (atomic number
= 11) is
Using noble-gas notation, sodium’s electron configuration can
be shortened to the form [Ne] 3 s 1 .
Exceptionsto predicted configurations
You can use theaufbau diagram to write correct ground-state electron
configurations](https://image.slidesharecdn.com/electronconfigurationlecturenotes-170316101902/85/Electron-configuration-lecture-notes-6-320.jpg)


![ Valenceelectrons are defined as electrons inthe atom’s outermost
orbitals—generally those orbitals associated with theatom’s
highest principal energy level.
Example:
SulfuratomS contains16 electrons,
Noble-gas notationof S is [Ne] 3S 2
3 p 4
Sulfur has six valence electrons.
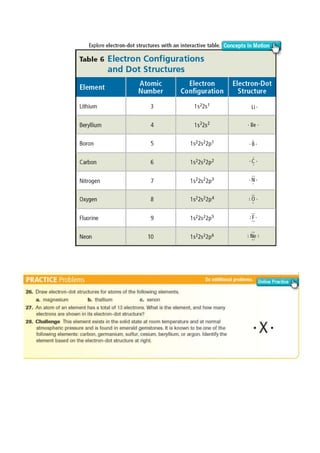
Electron-dot structures(Lewisstructure) :
Consists of the element’ssymbol,surrounded bydots representing
all of the atom’s valenceelectrons](https://image.slidesharecdn.com/electronconfigurationlecturenotes-170316101902/85/Electron-configuration-lecture-notes-9-320.jpg)