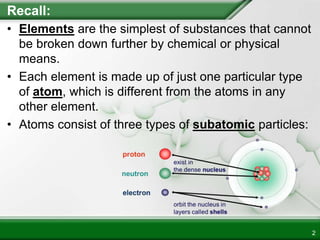
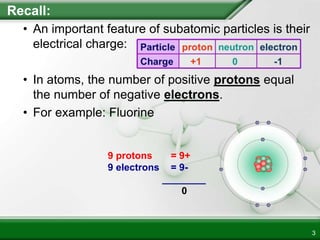
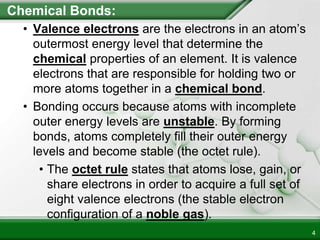
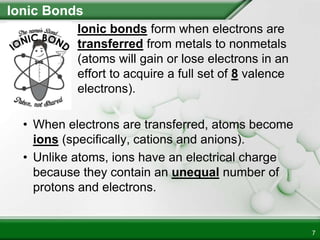
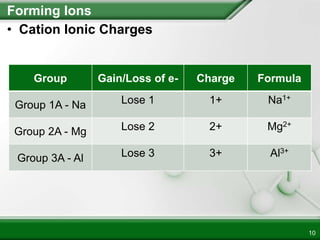
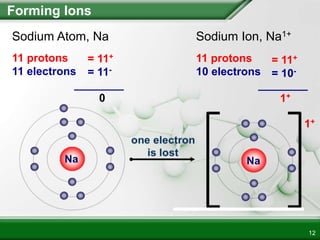
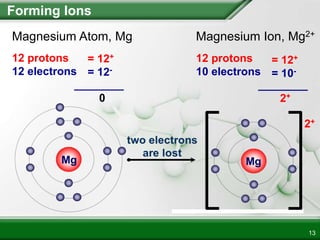
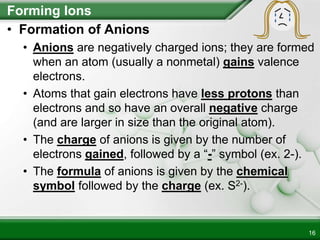
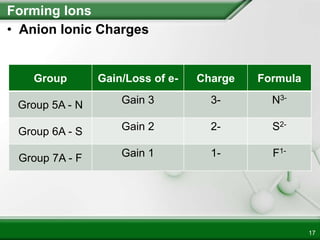
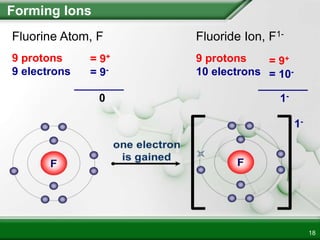
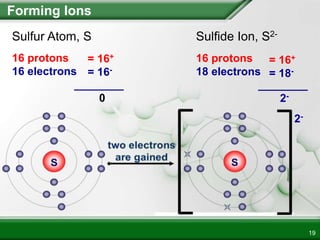
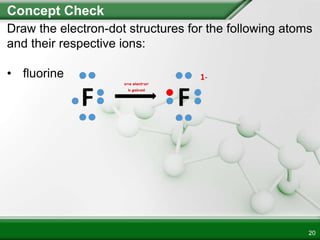
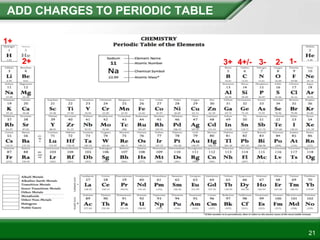
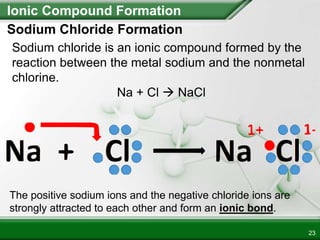
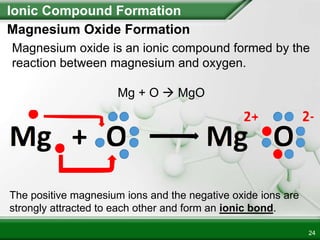
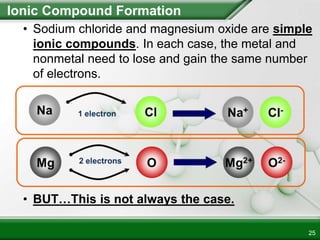
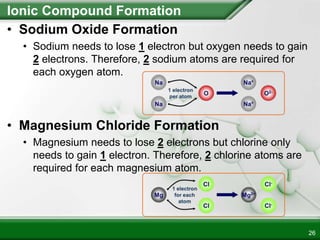
Ionic compounds form when ions bond through electrostatic attraction. Metals form cations by losing electrons to achieve a full outer shell, while nonmetals form anions by gaining electrons. Cations and anions are attracted due to their opposite charges. Ionic compounds have high melting points, are crystalline solids, and dissolve in water due to the separation of ions. They do not conduct electricity as solids but do so as liquids or dissolved solutions.