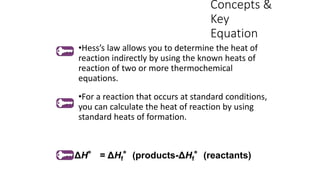
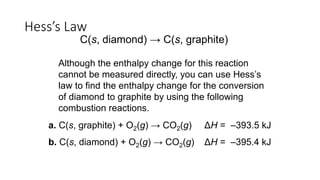
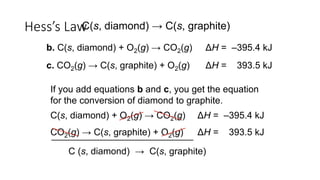
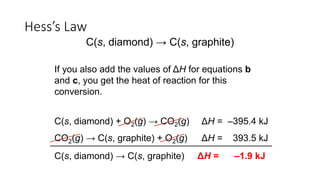
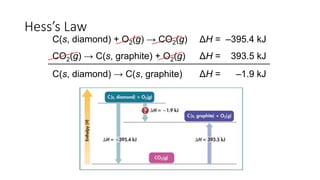
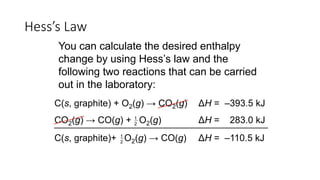
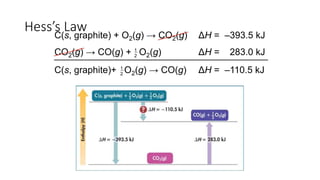
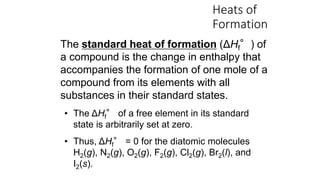
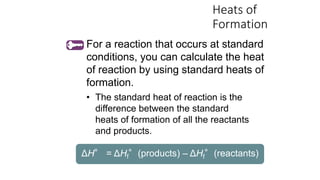
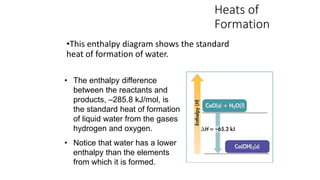
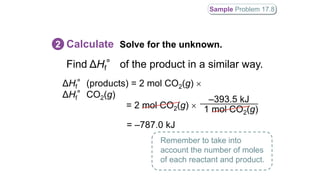
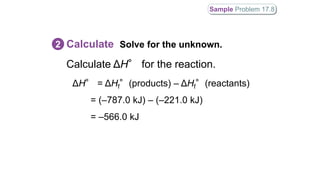
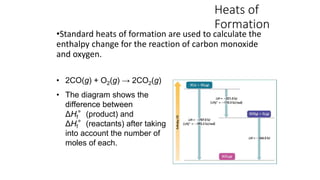
Hess's law states that the total enthalpy change for a reaction is independent of the pathway taken. It allows calculation of enthalpy changes that cannot be measured directly by combining thermochemical equations. The standard heat of reaction (ΔH°) can be calculated from standard heats of formation (ΔHf°) of reactants and products. Hess's law and heats of formation are useful for determining enthalpy changes that cannot be measured in the laboratory.




















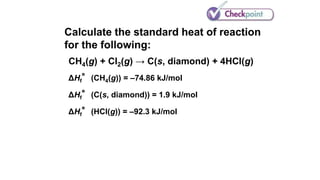
![Calculate the standard heat of reaction
for the following:
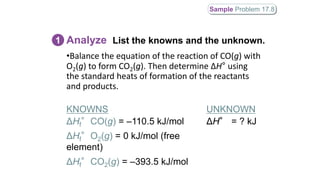
CH4(g) + Cl2(g) → C(s, diamond) + 4HCl(g)
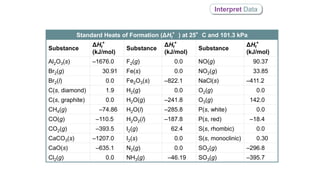
ΔHf°(CH4(g)) = –74.86 kJ/mol
ΔHf°(C(s, diamond)) = 1.9 kJ/mol
ΔHf°(HCl(g)) = –92.3 kJ/mol
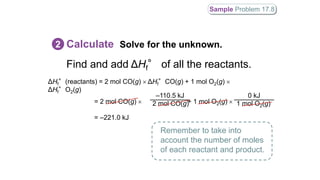
ΔHf°(reactants) = [1 mol CH4(g) ΔHf°CH4(g)] + [1 mol Cl2 ΔHf°Cl2(g)]
= –74.86 kJ + 0.0 kJ = –74.86 kJ
ΔHf°(products) = [1 mol C(s) ΔHf°C(s, diamond)] + [4 mol HCl
ΔHf°HCl(g)]
= 1.9 kJ + (4 –92.3 kJ) = –367.3 kJ
ΔH° = ΔHf°(products) – ΔHf°(reactants) = –367.3 kJ – (–74.86 kJ) = –](https://image.slidesharecdn.com/hessslaw-200324053204/85/Hess-s-law-40-320.jpg)