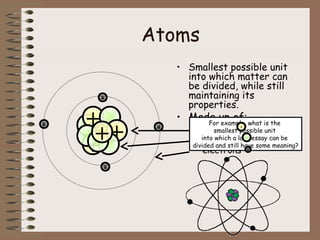
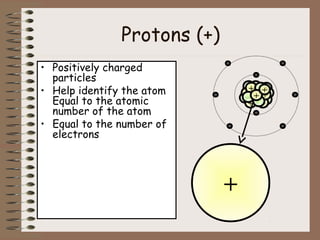
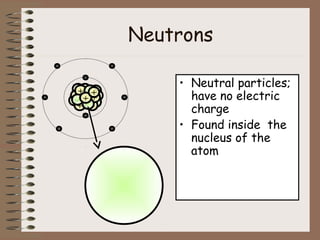
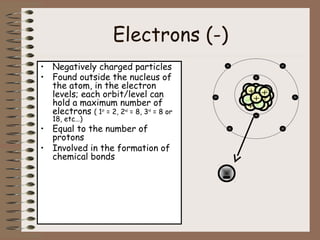
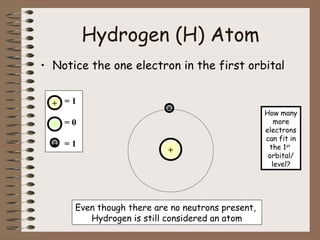
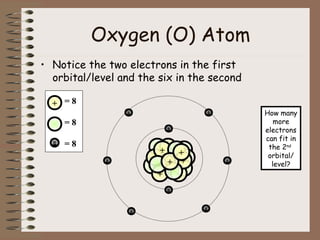
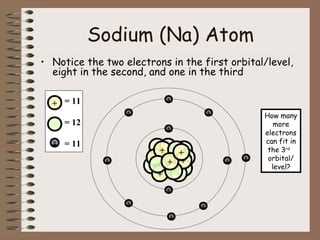
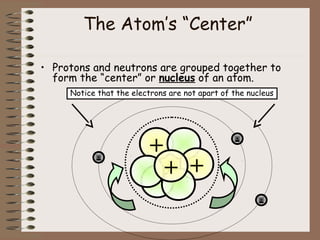
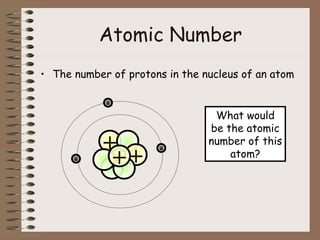
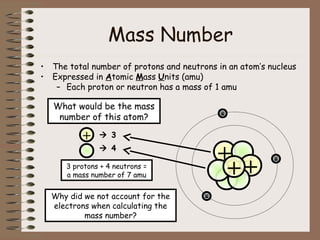
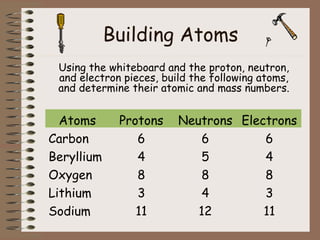
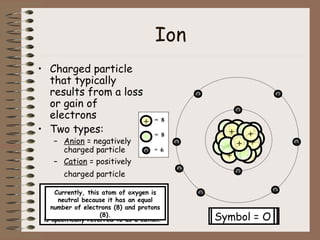
The document discusses the basic building blocks of matter at the atomic level. It explains that atoms are made up of protons, neutrons, and electrons. Protons are positively charged and found in the atom's nucleus along with neutrons, which have no charge. Electrons are negatively charged and orbit the nucleus. It provides examples of hydrogen, oxygen, and sodium atoms, showing their proton, neutron, and electron composition. The atomic number is the number of protons, while the mass number includes protons and neutrons. Atoms can gain or lose electrons to become ions that are electrically charged.