The document discusses several periodic trends, including how atomic radius, ionic radius, ionization energy, and electronegativity change across periods and down groups in the periodic table.
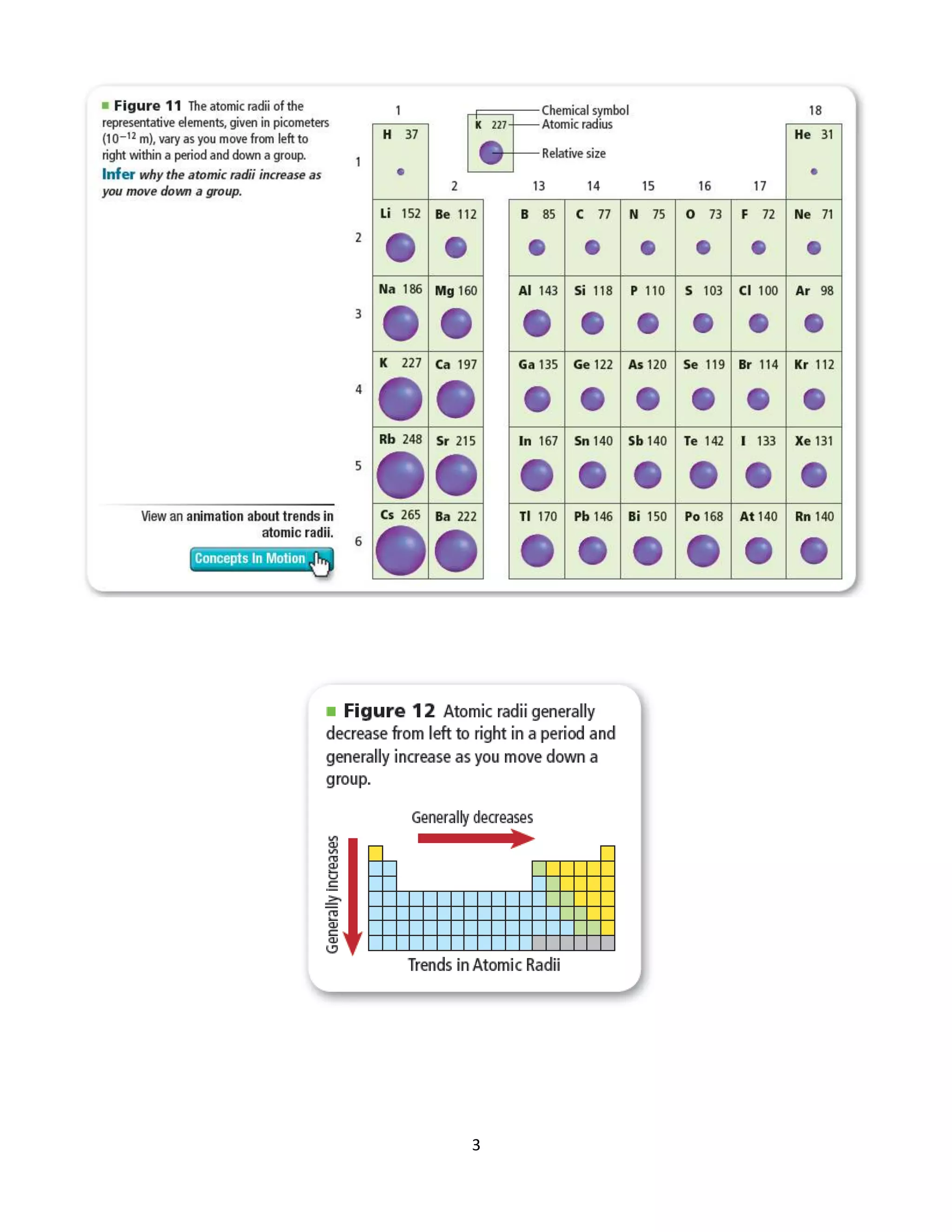
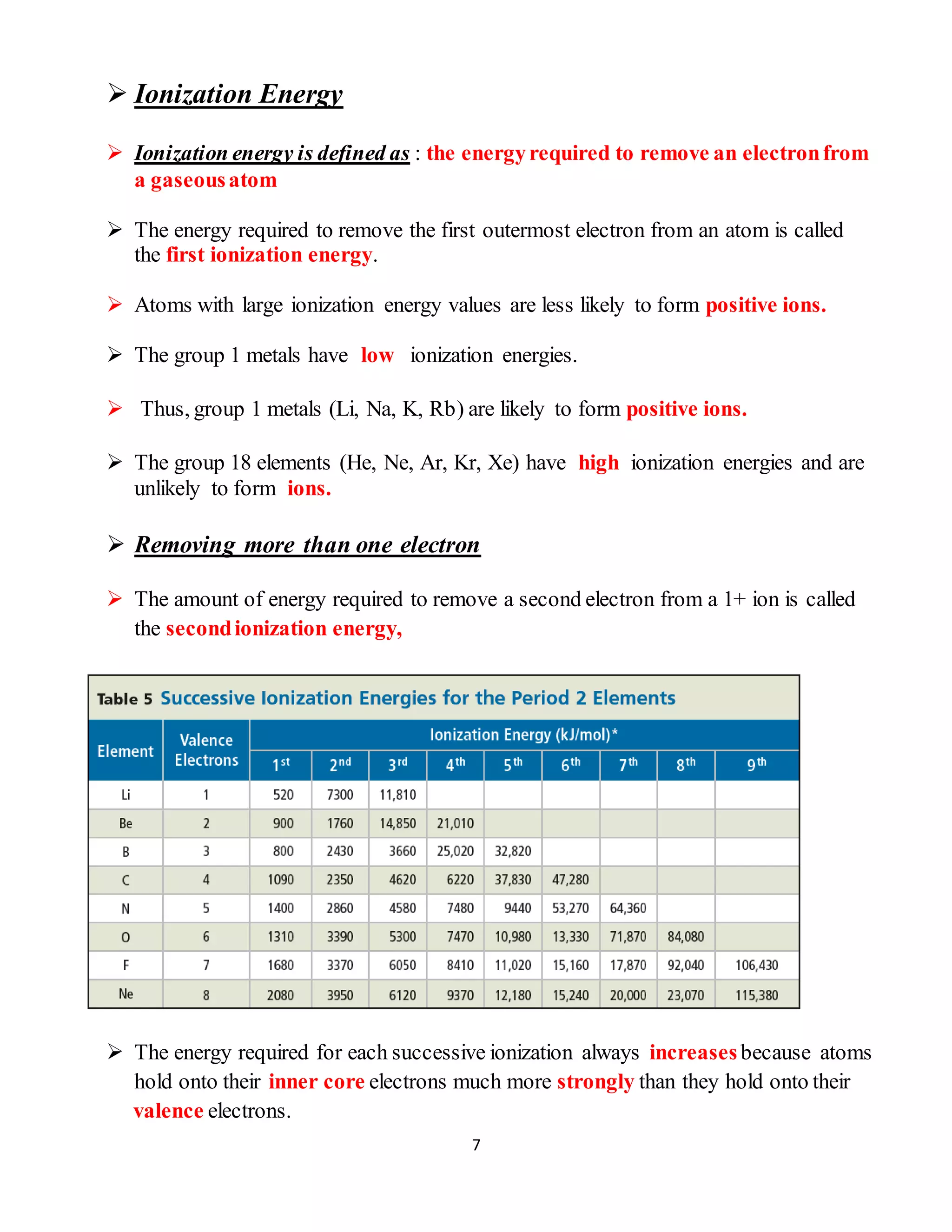
[1] Atomic radius decreases and ionization energy increases when moving across a period as nuclear charge increases, while trends are opposite when moving down a group as additional energy levels are added.
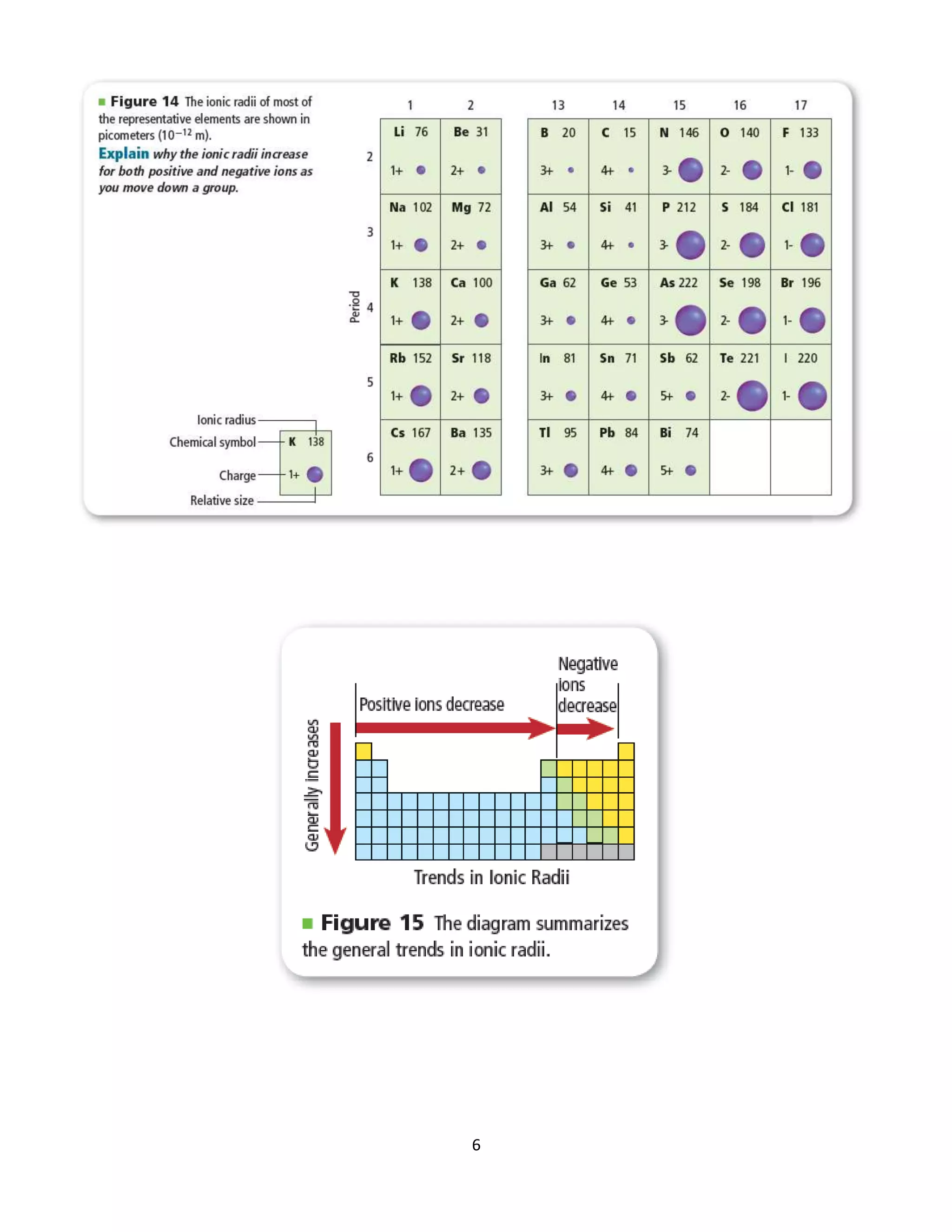
[2] Ionic radius follows the same trends as atomic radius, decreasing across periods and increasing down groups.
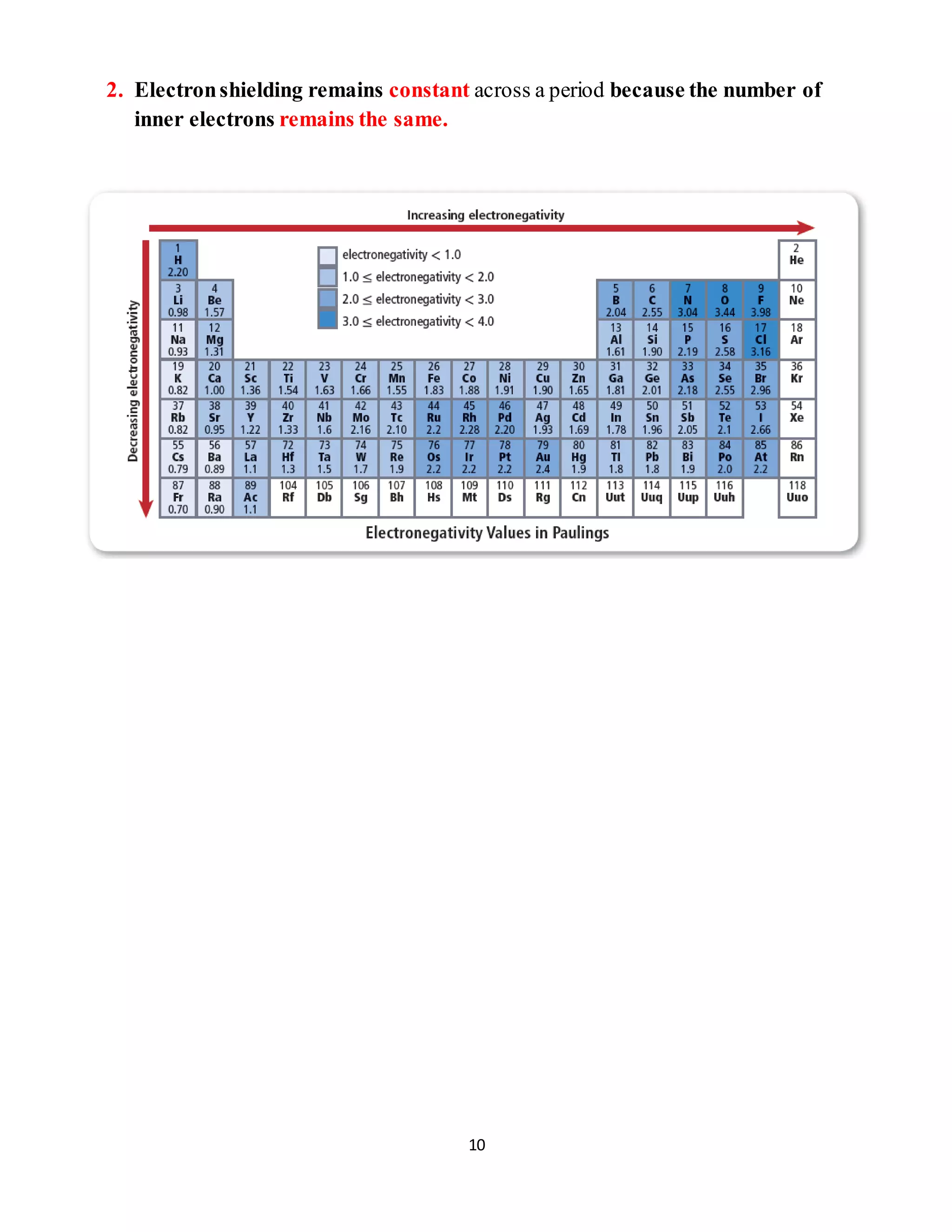
[3] Electronegativity also decreases down groups as the distance between nucleus and electrons increases, but increases across periods as nuclear charge rises.