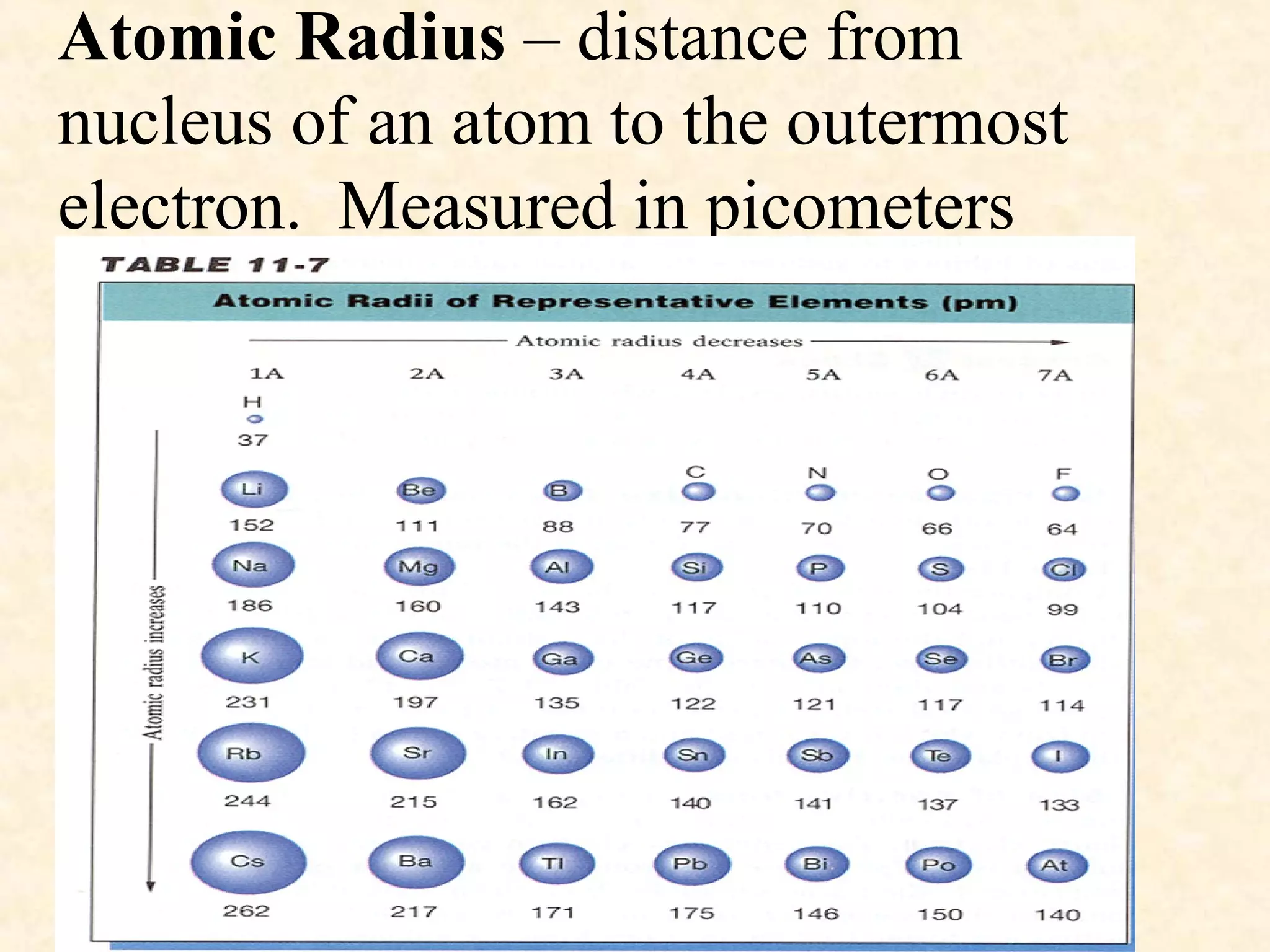
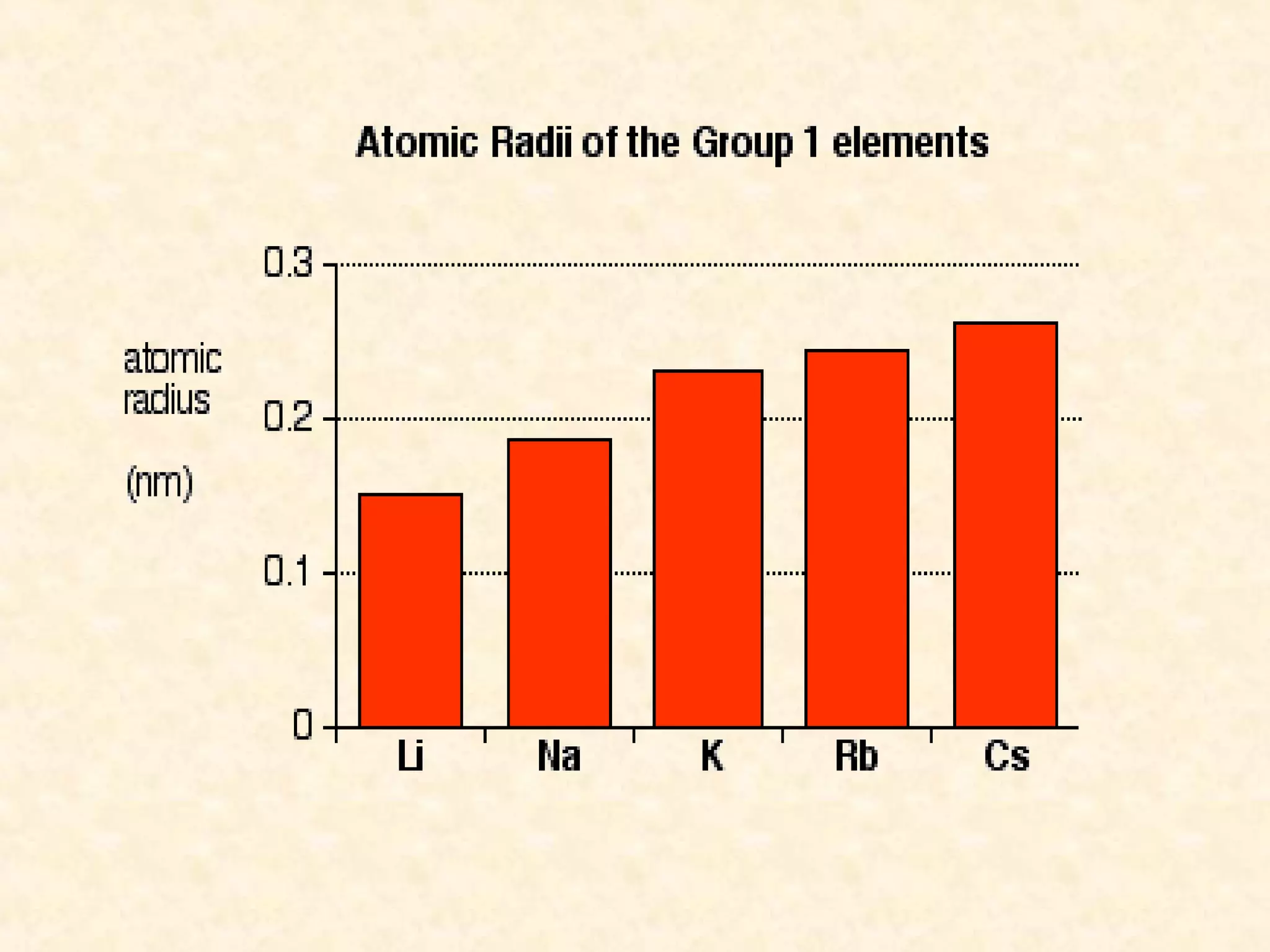
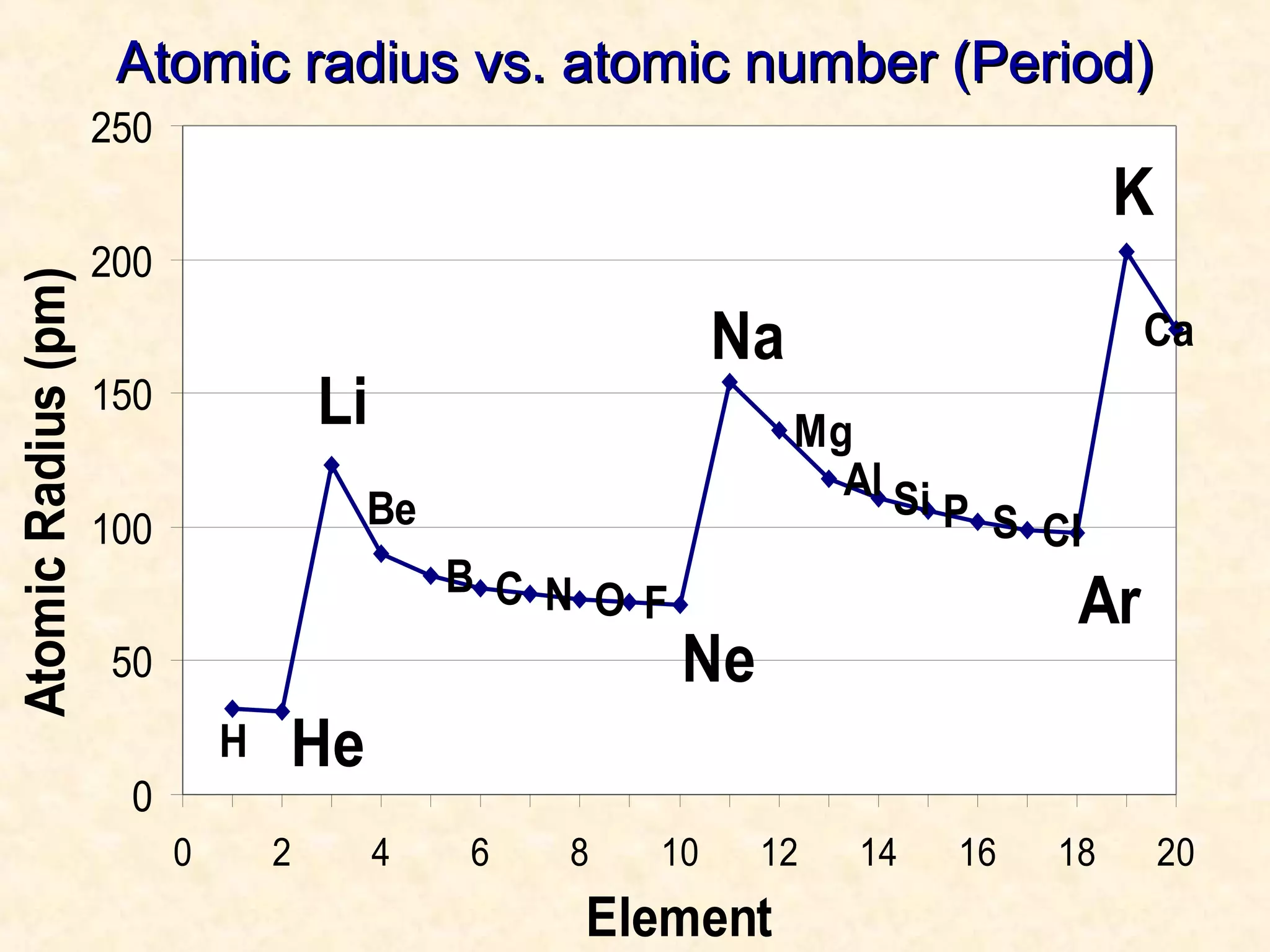
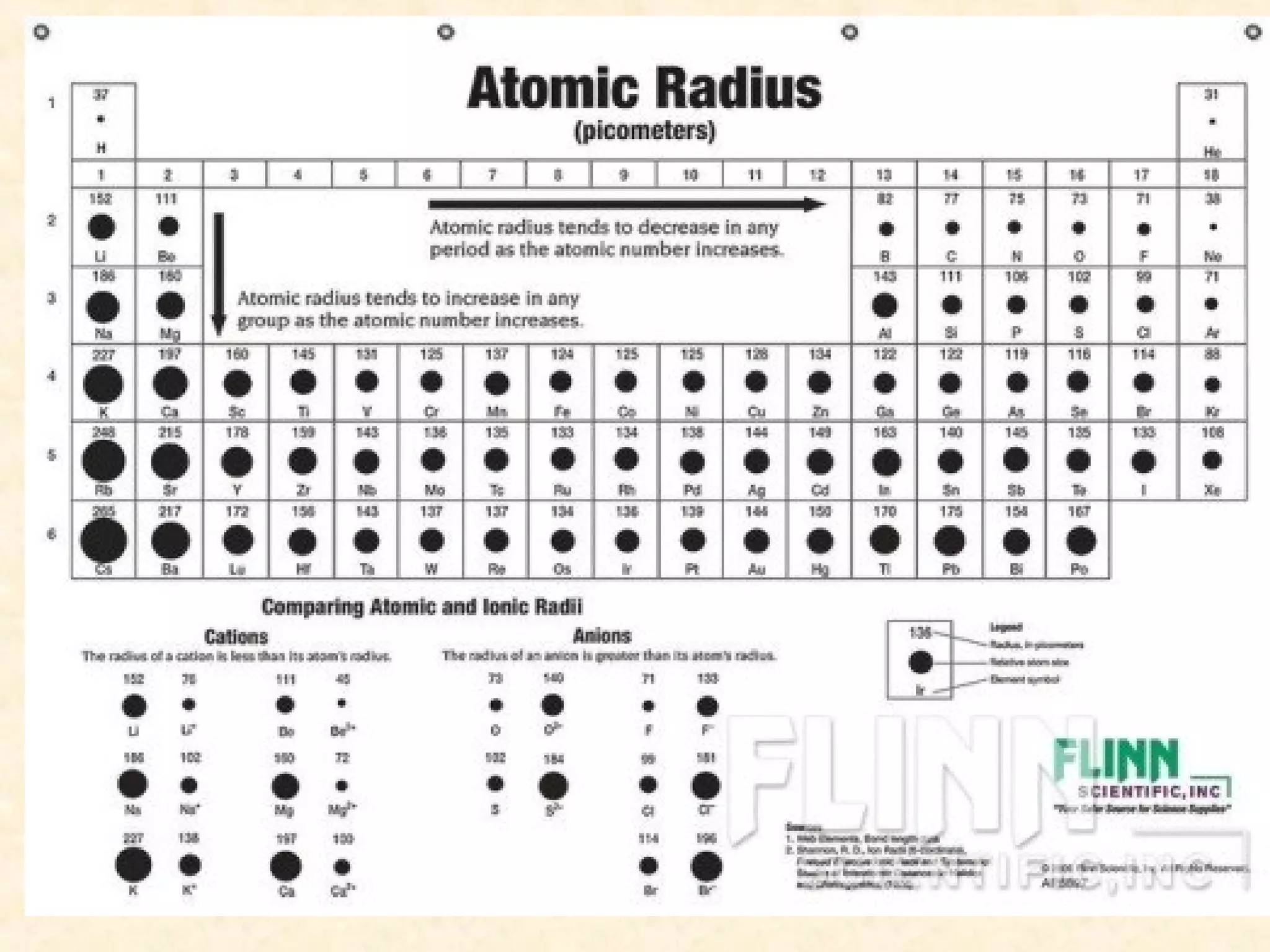
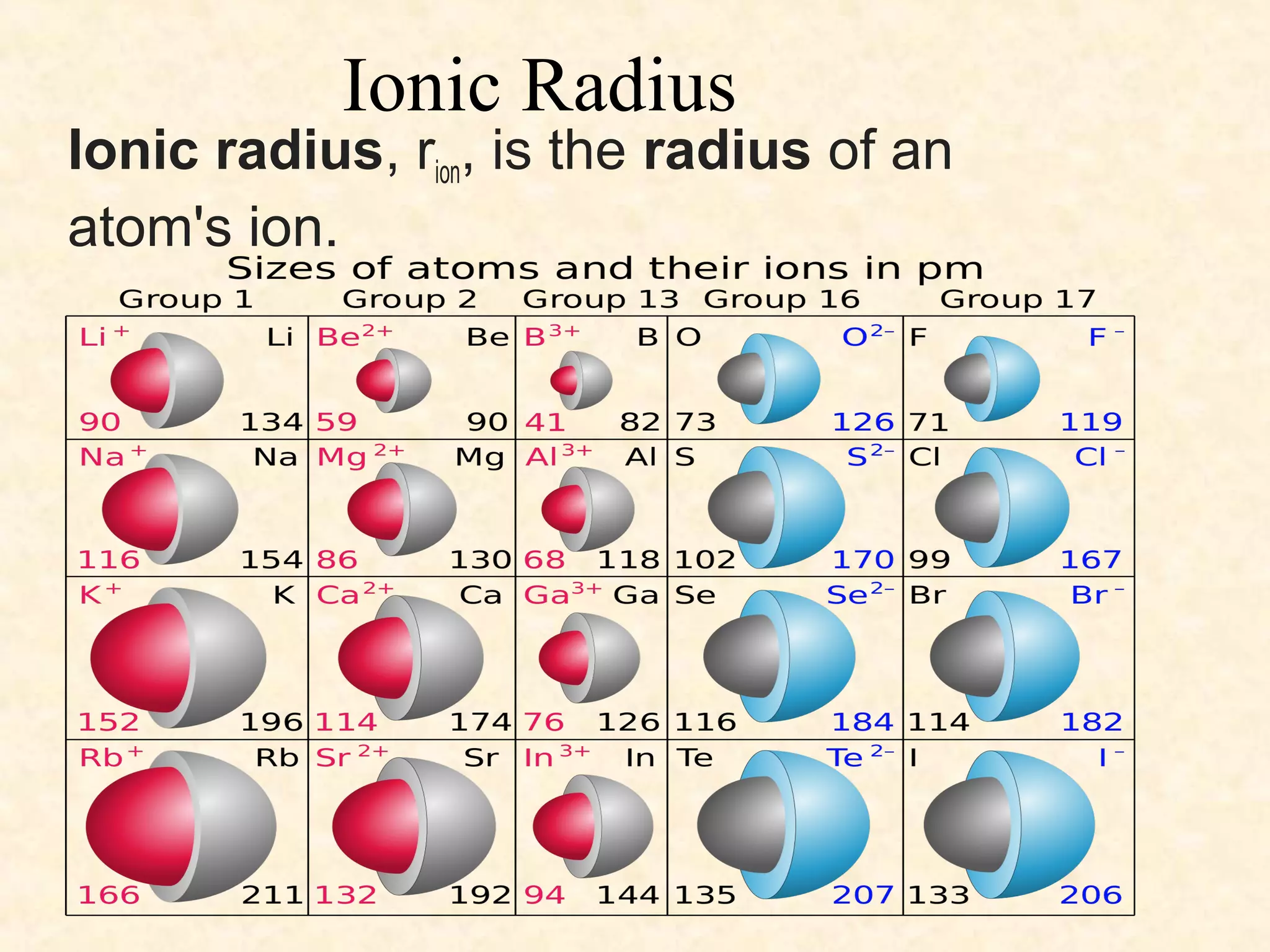
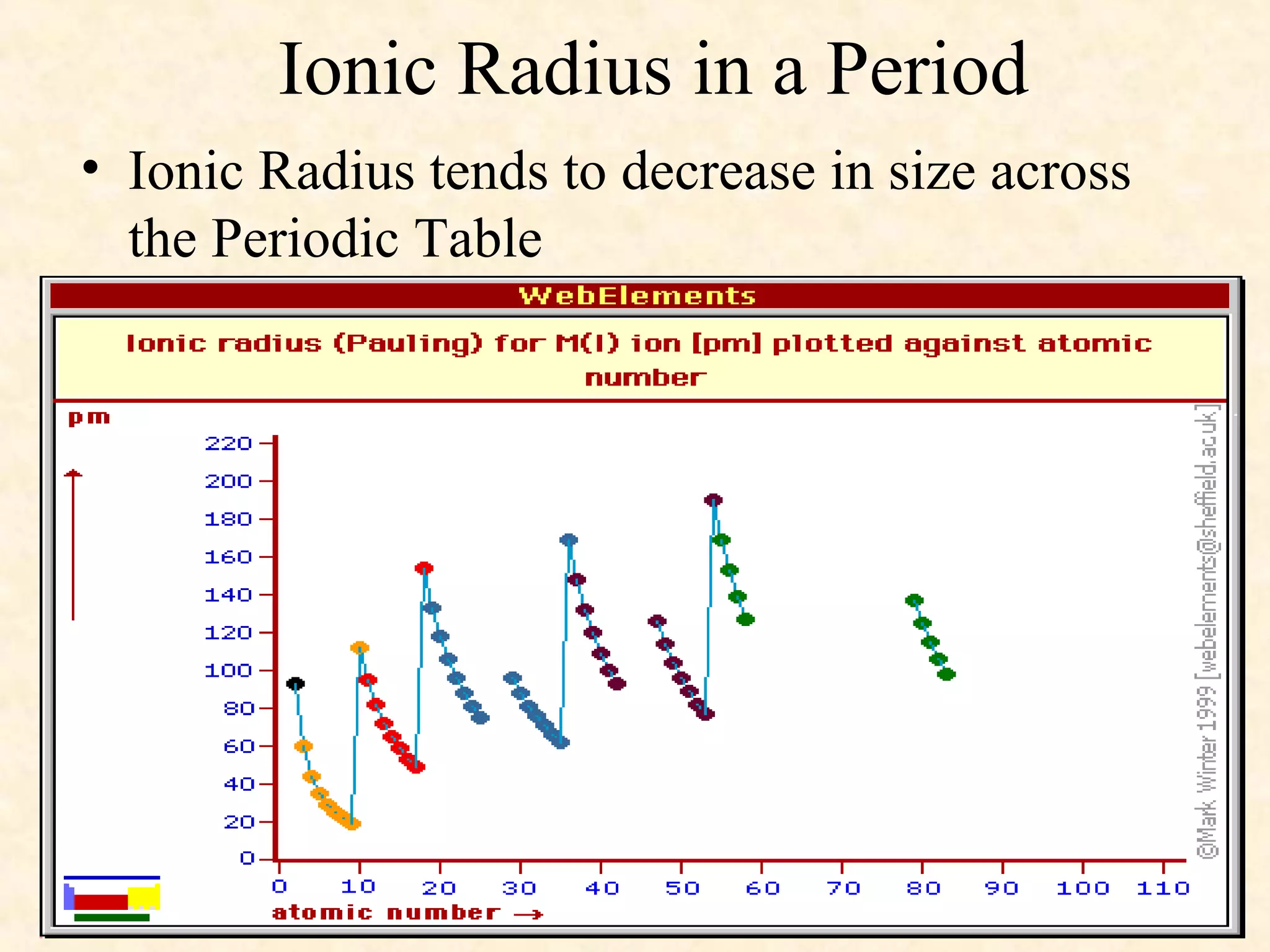
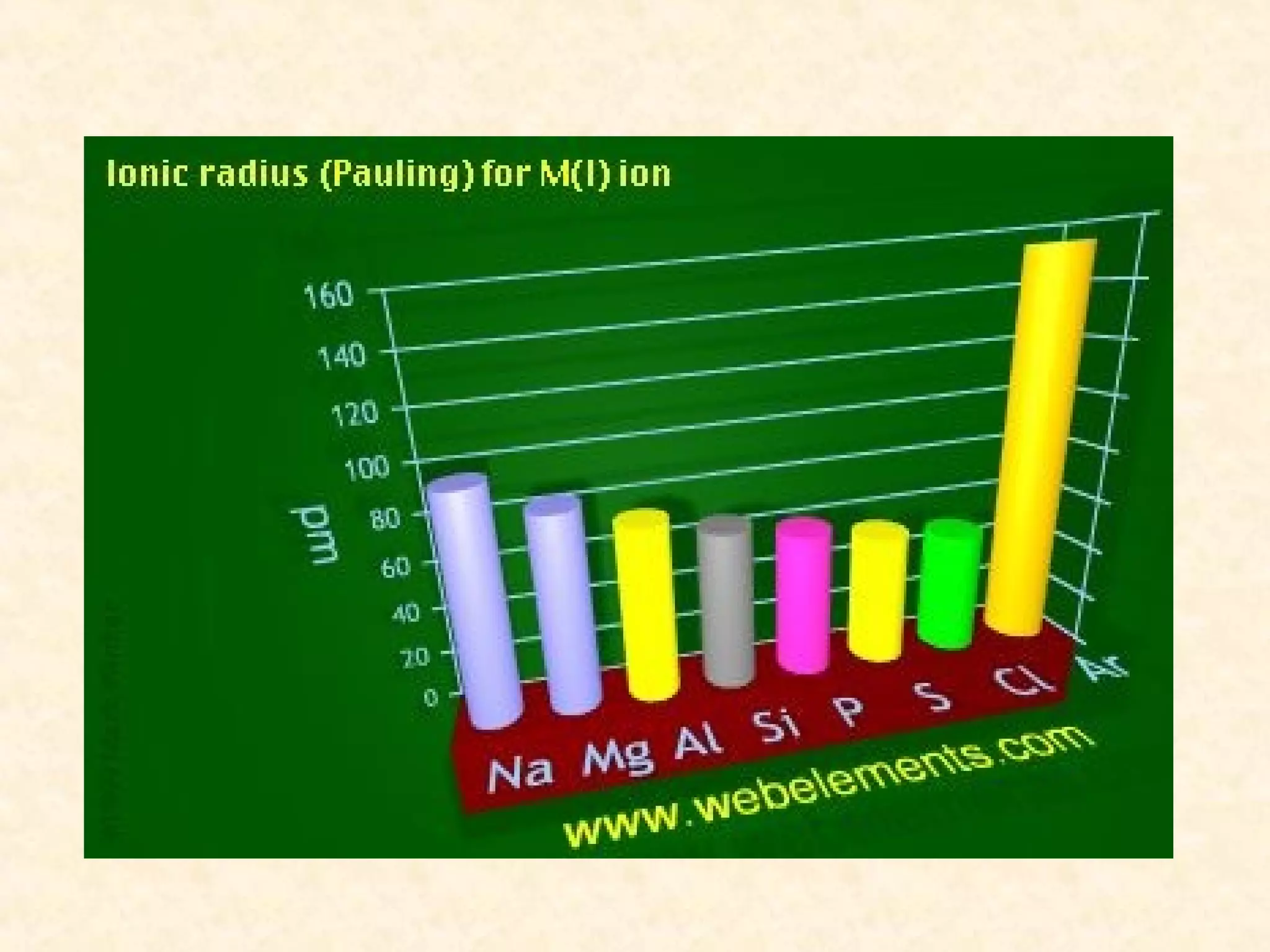
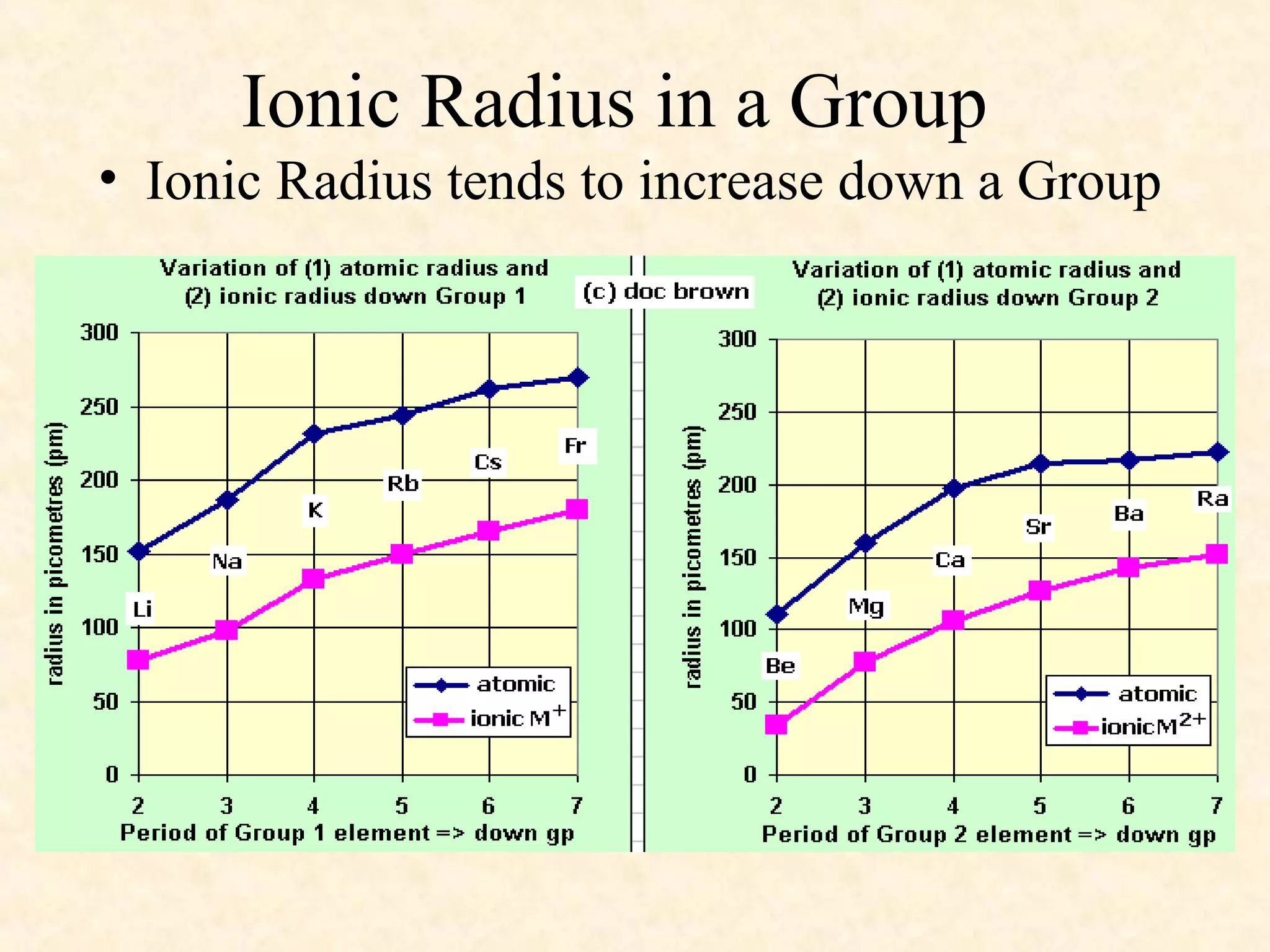
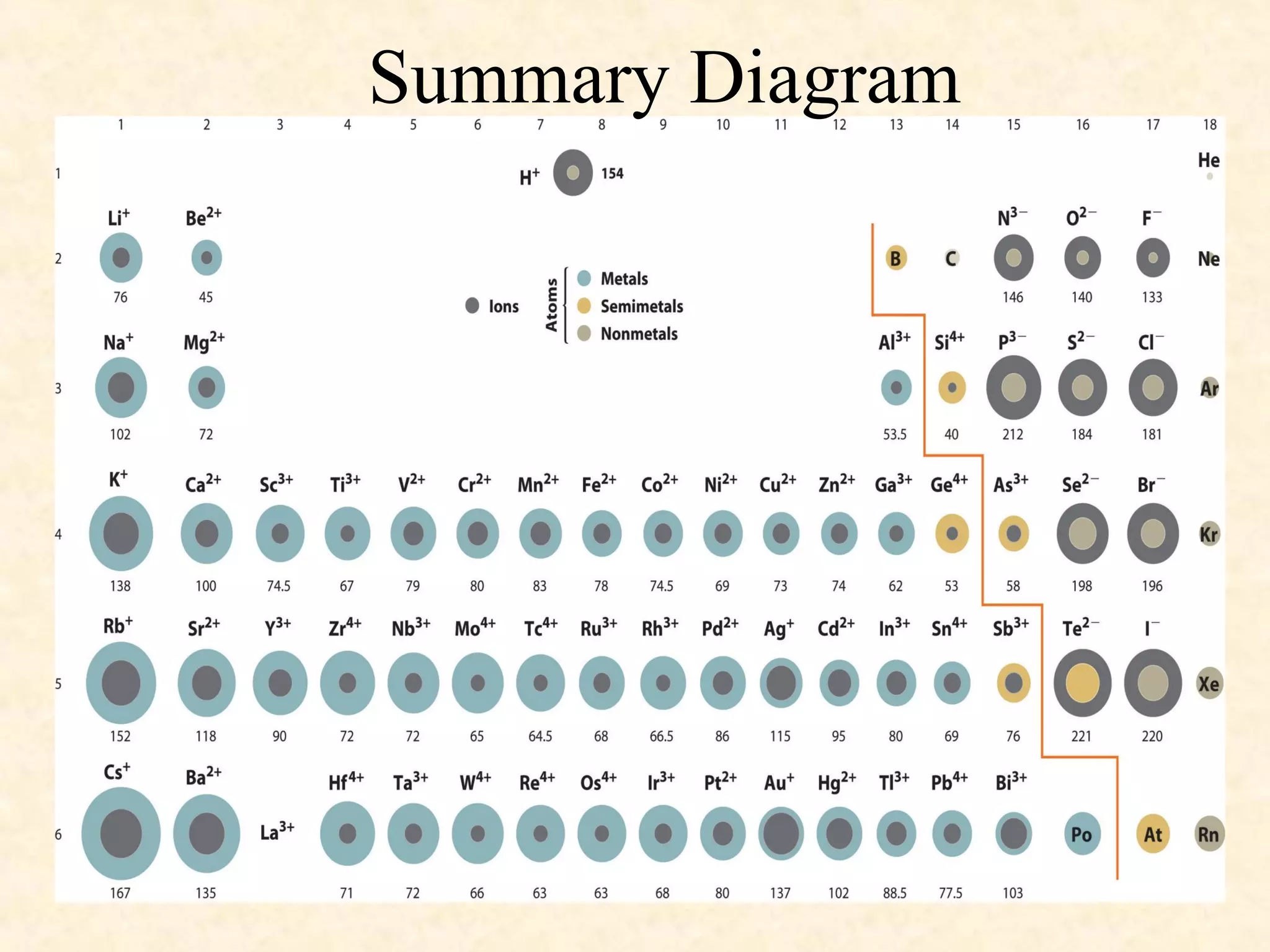
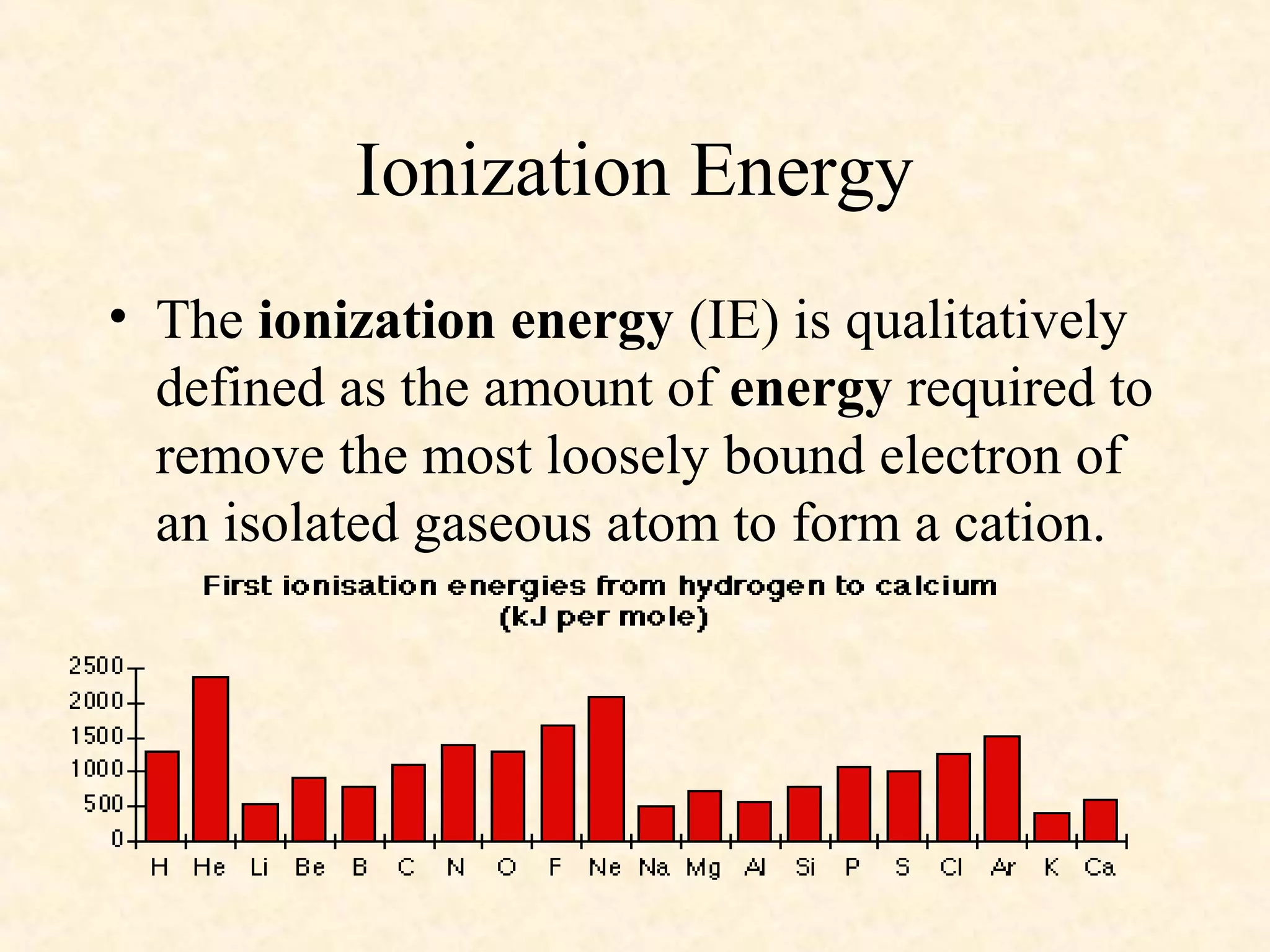
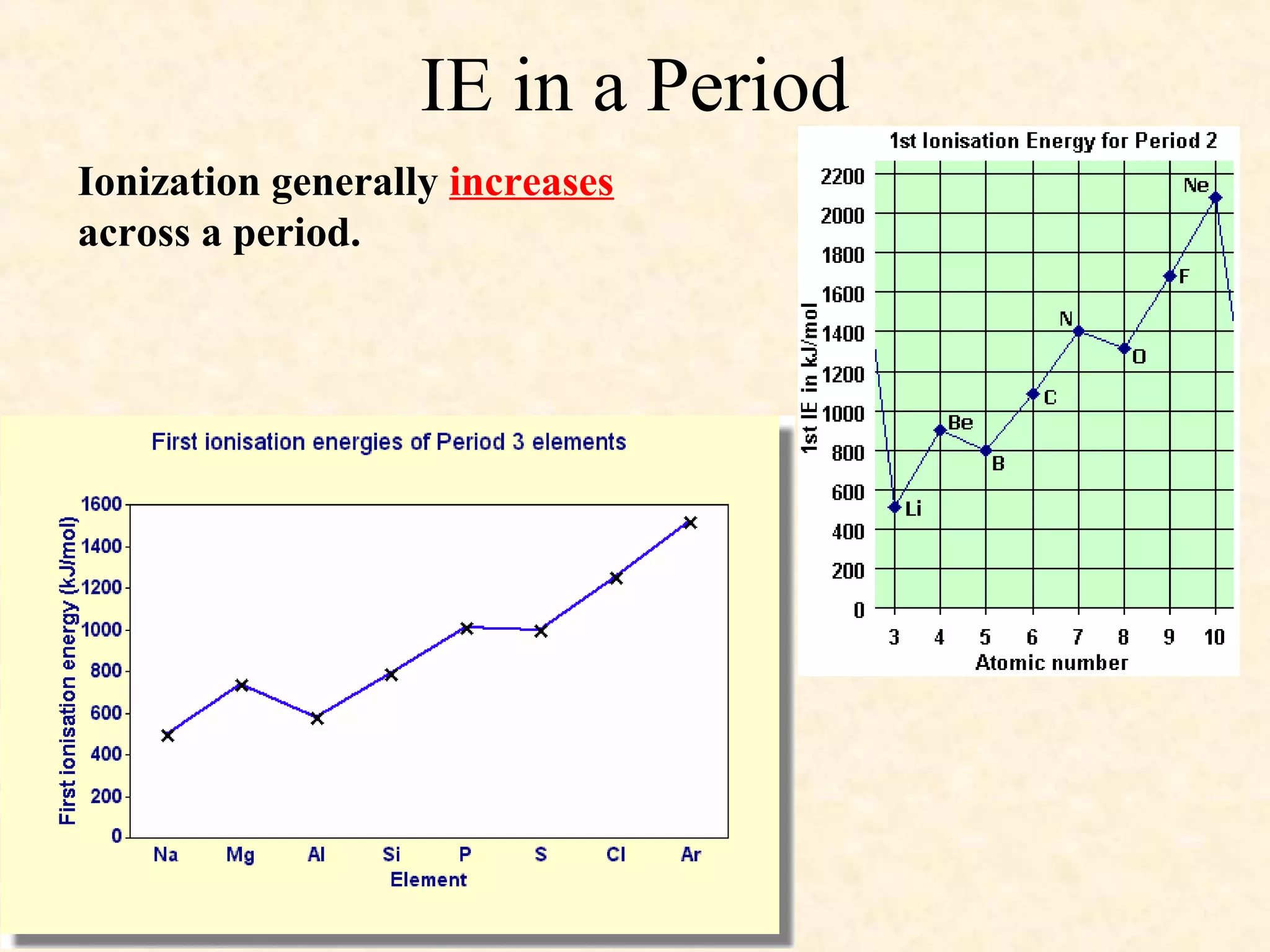
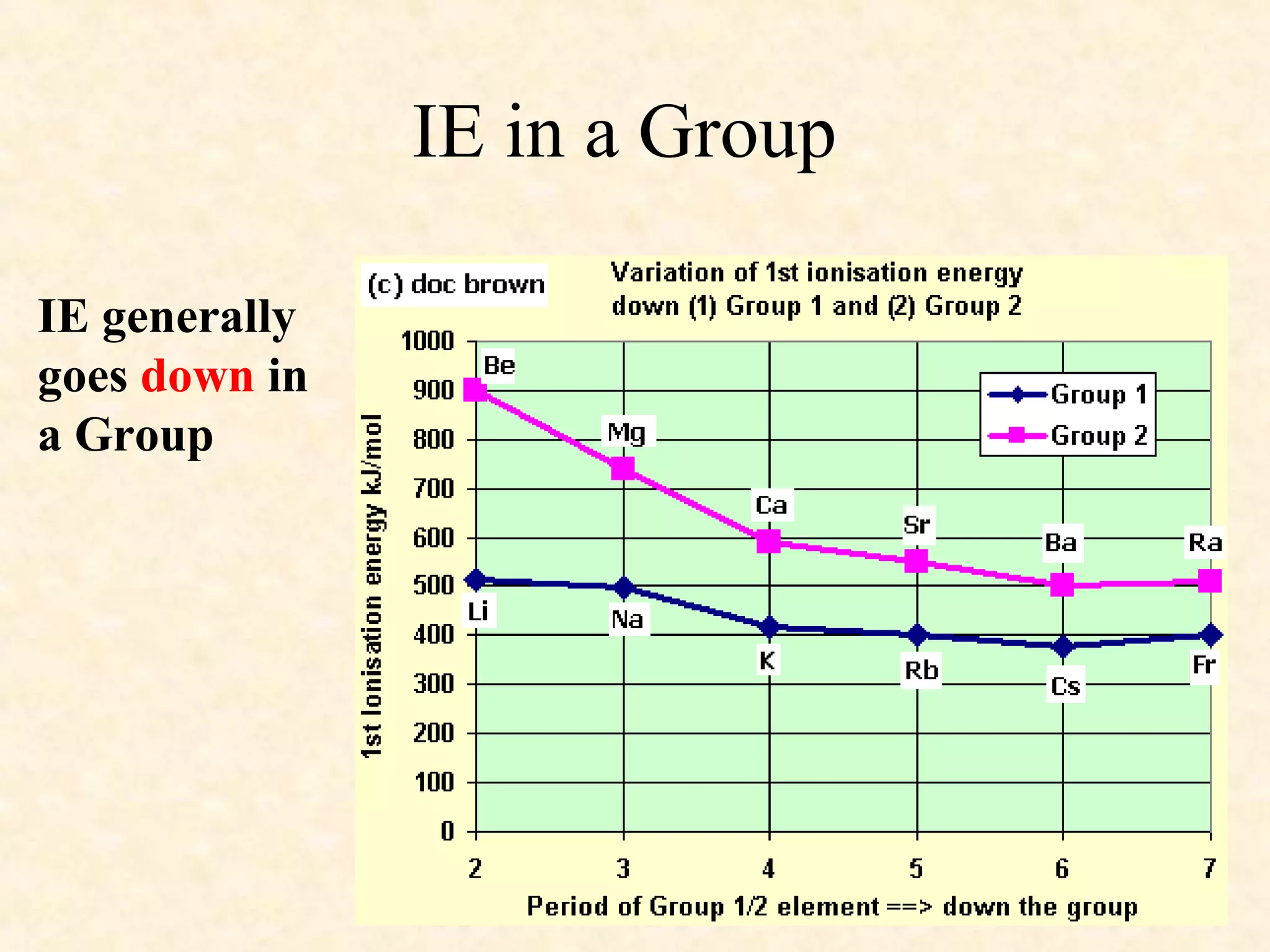
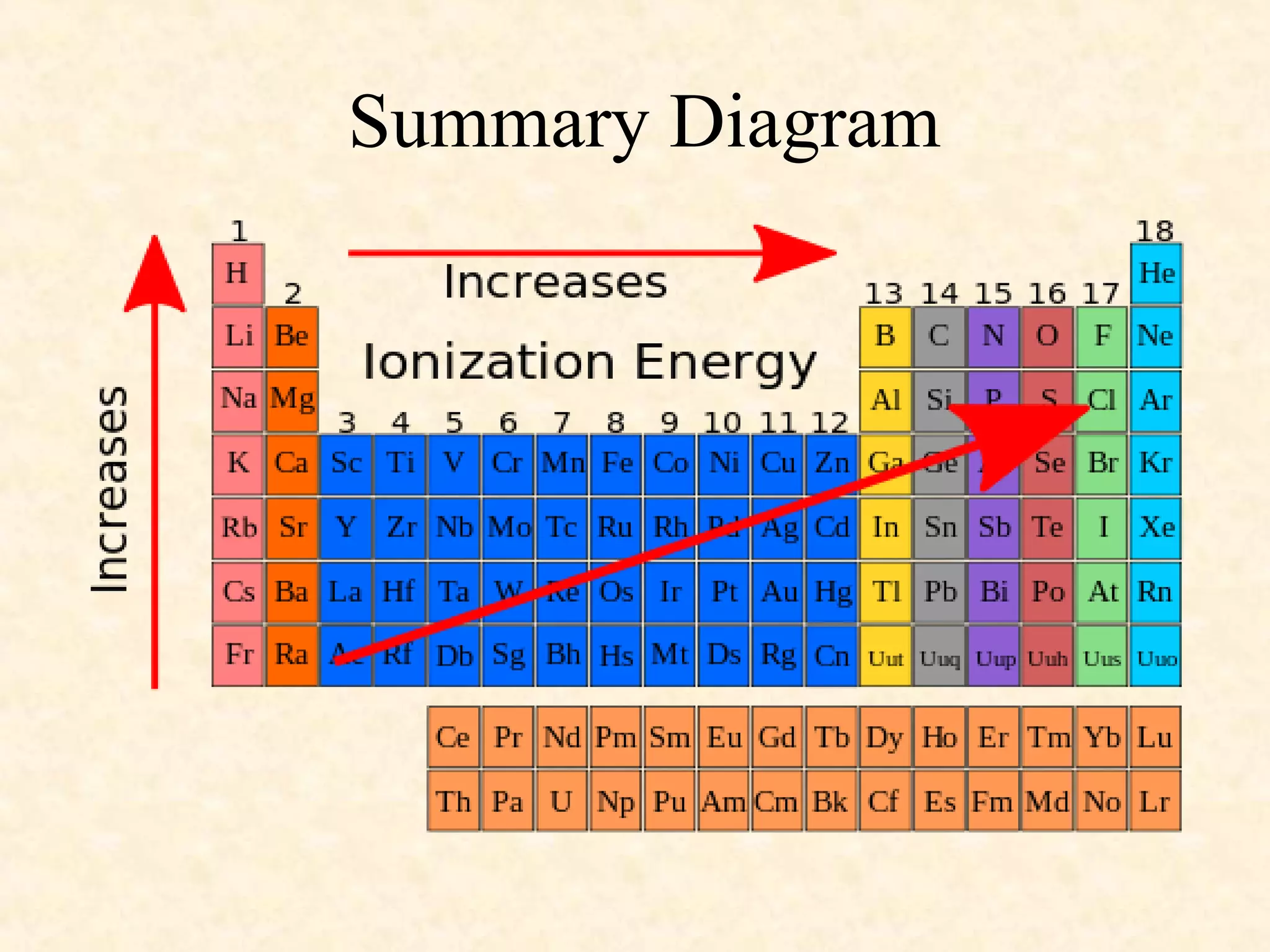
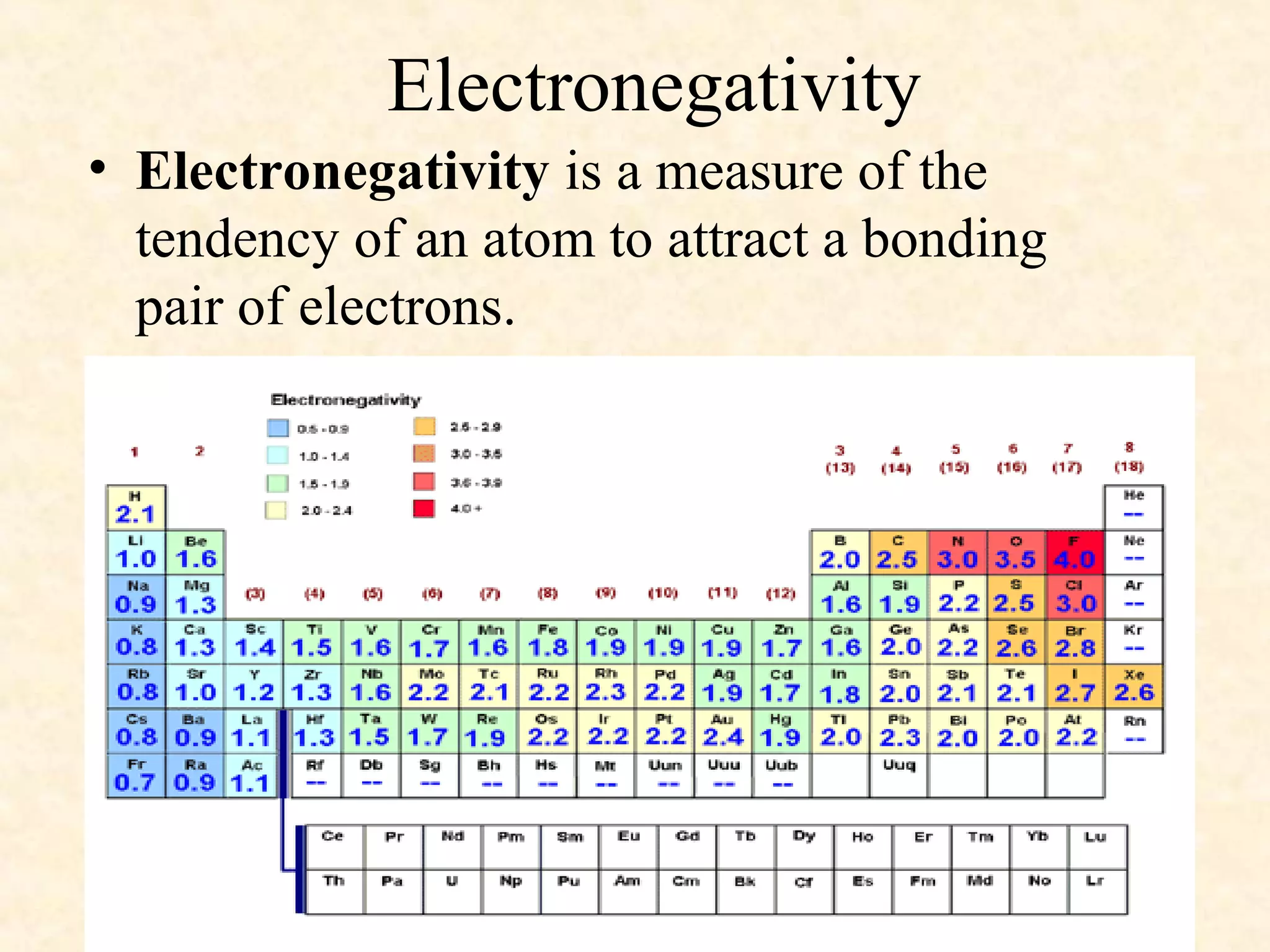
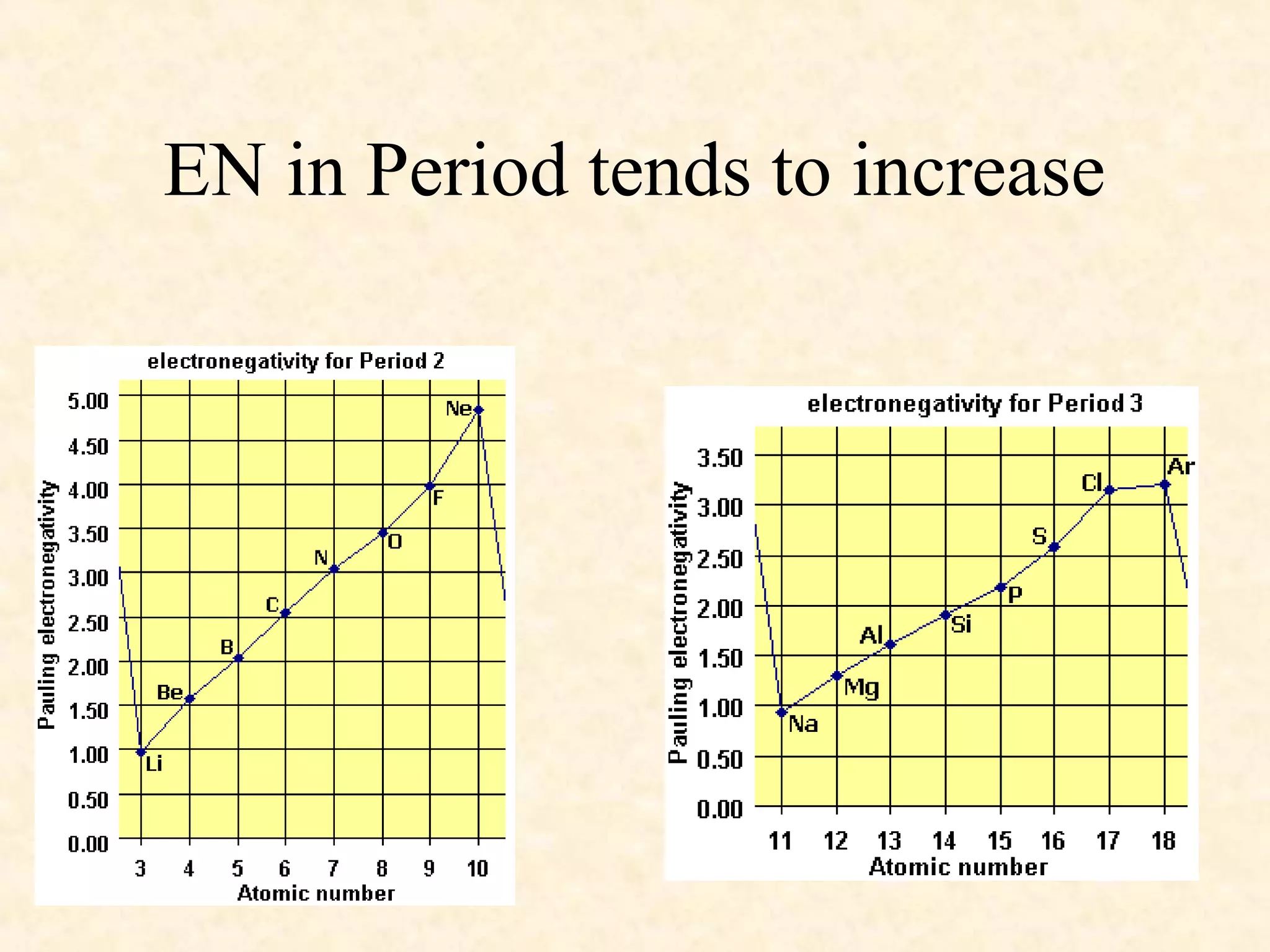
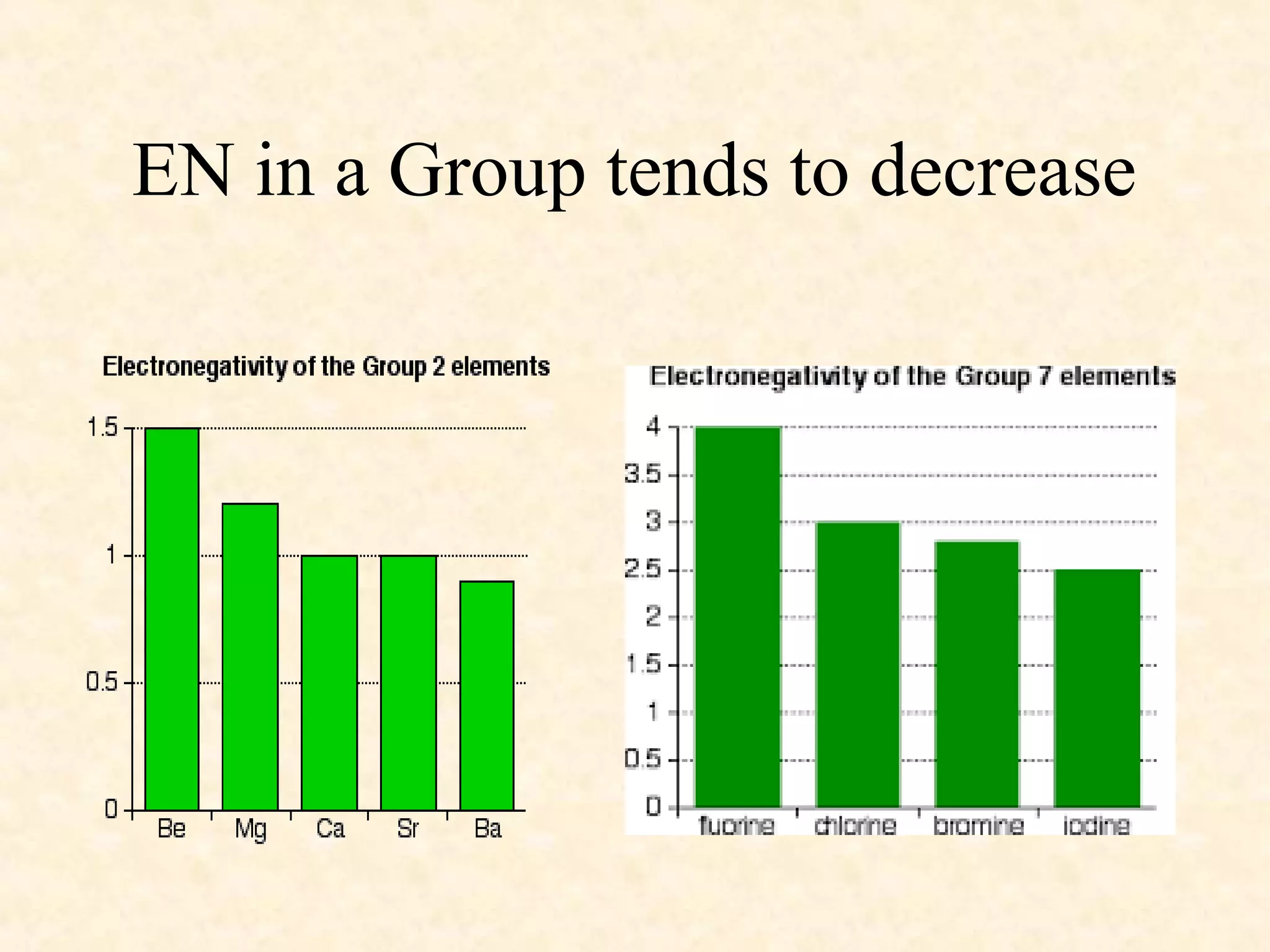
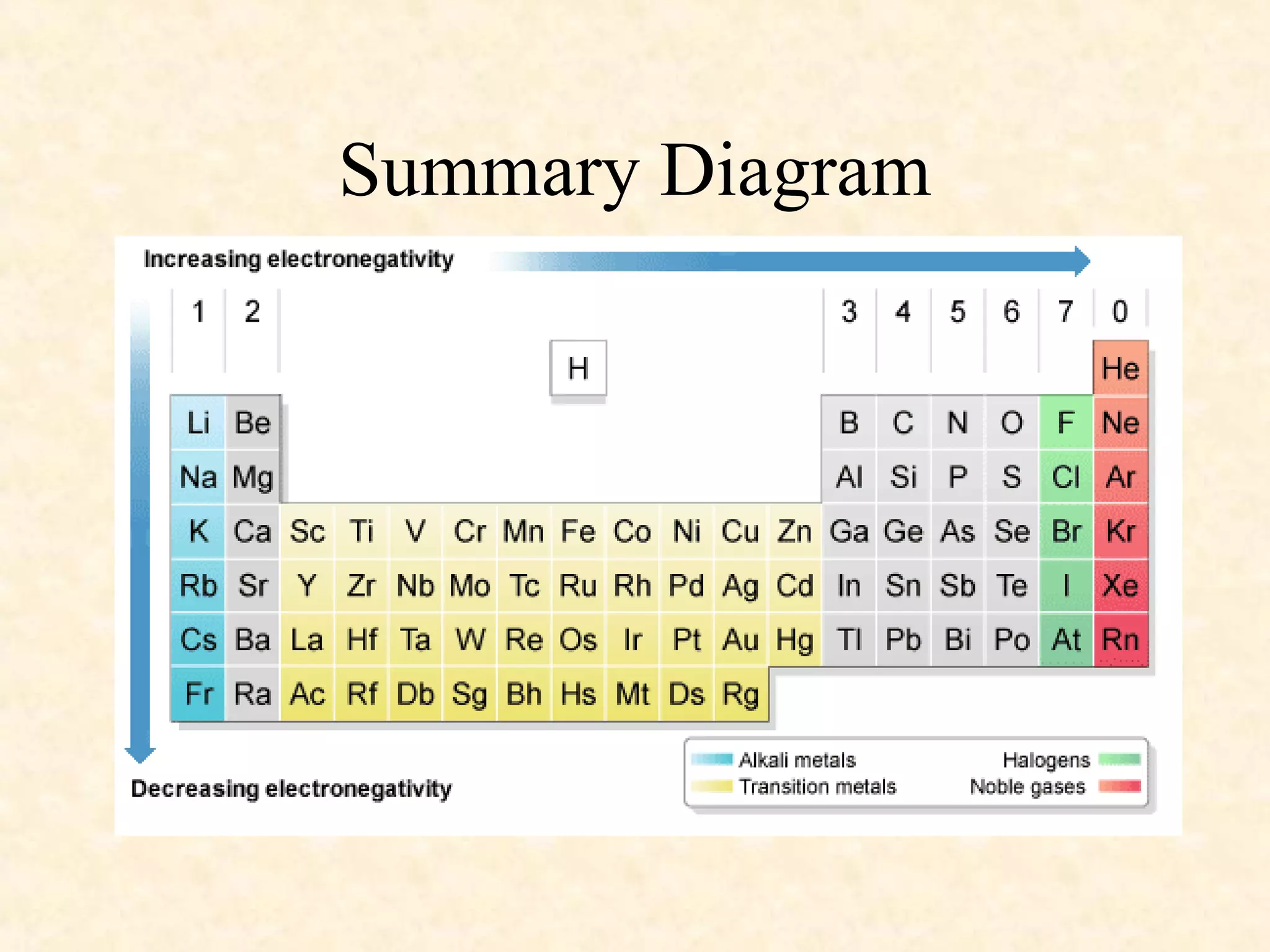
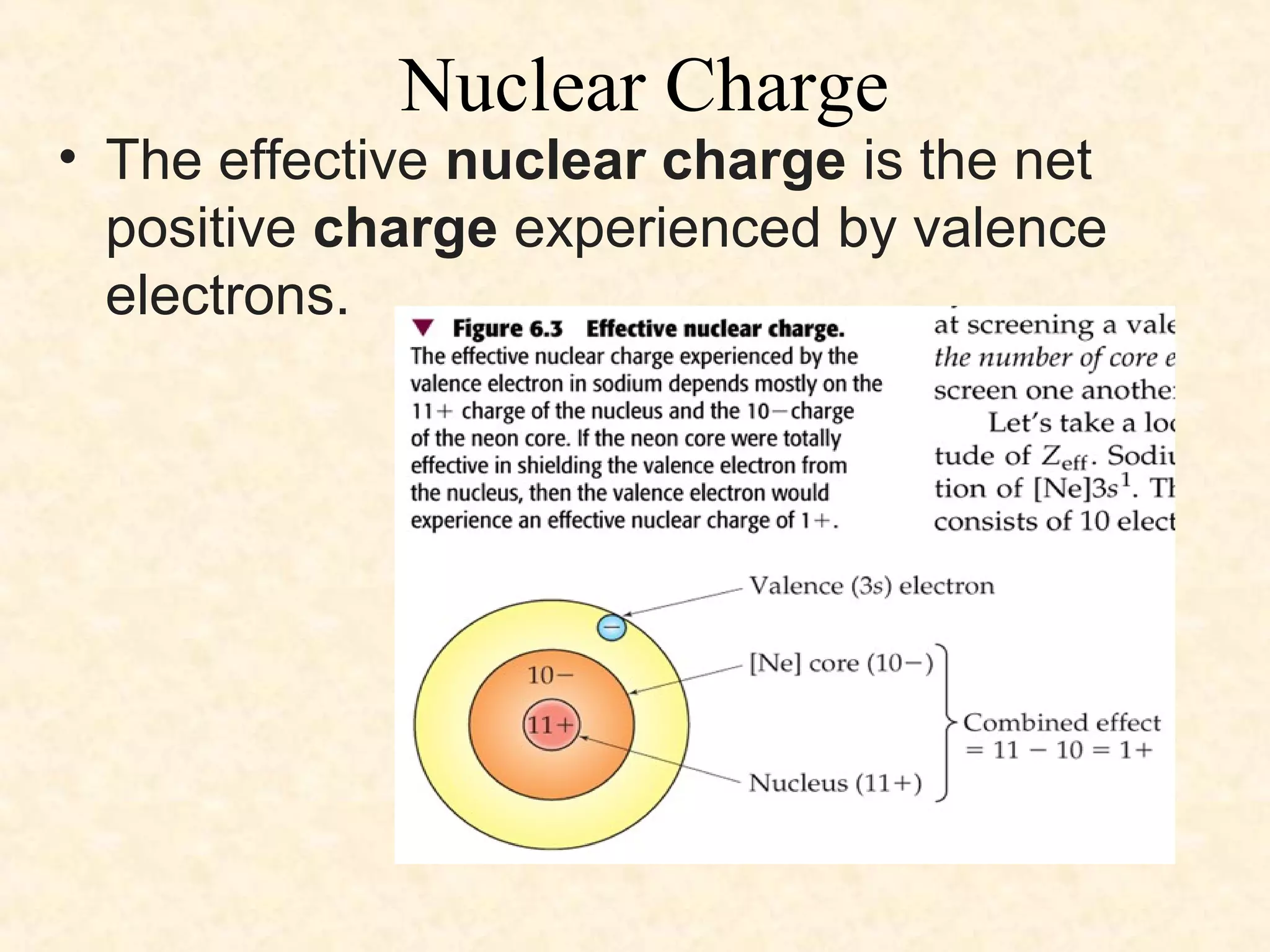
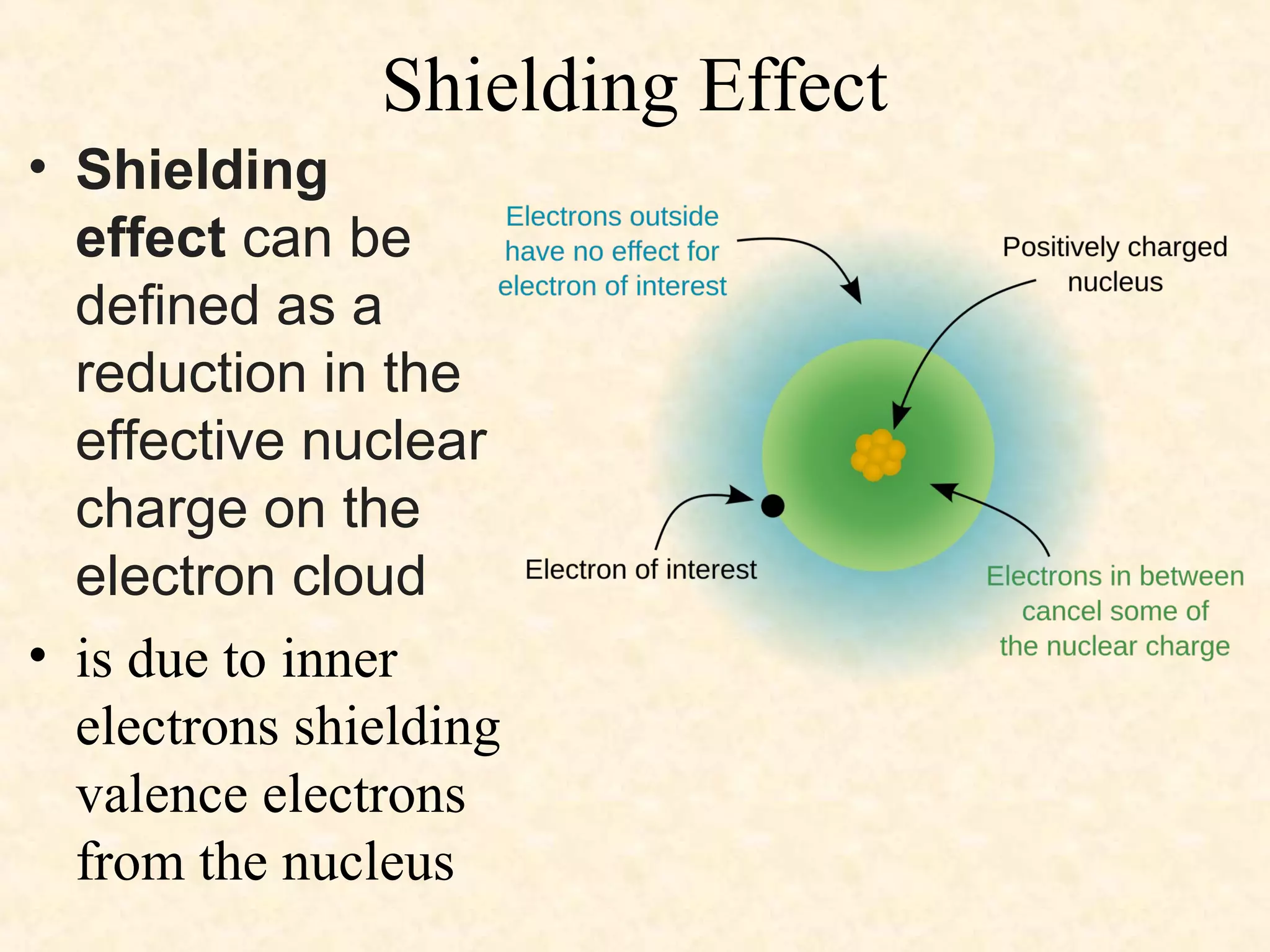
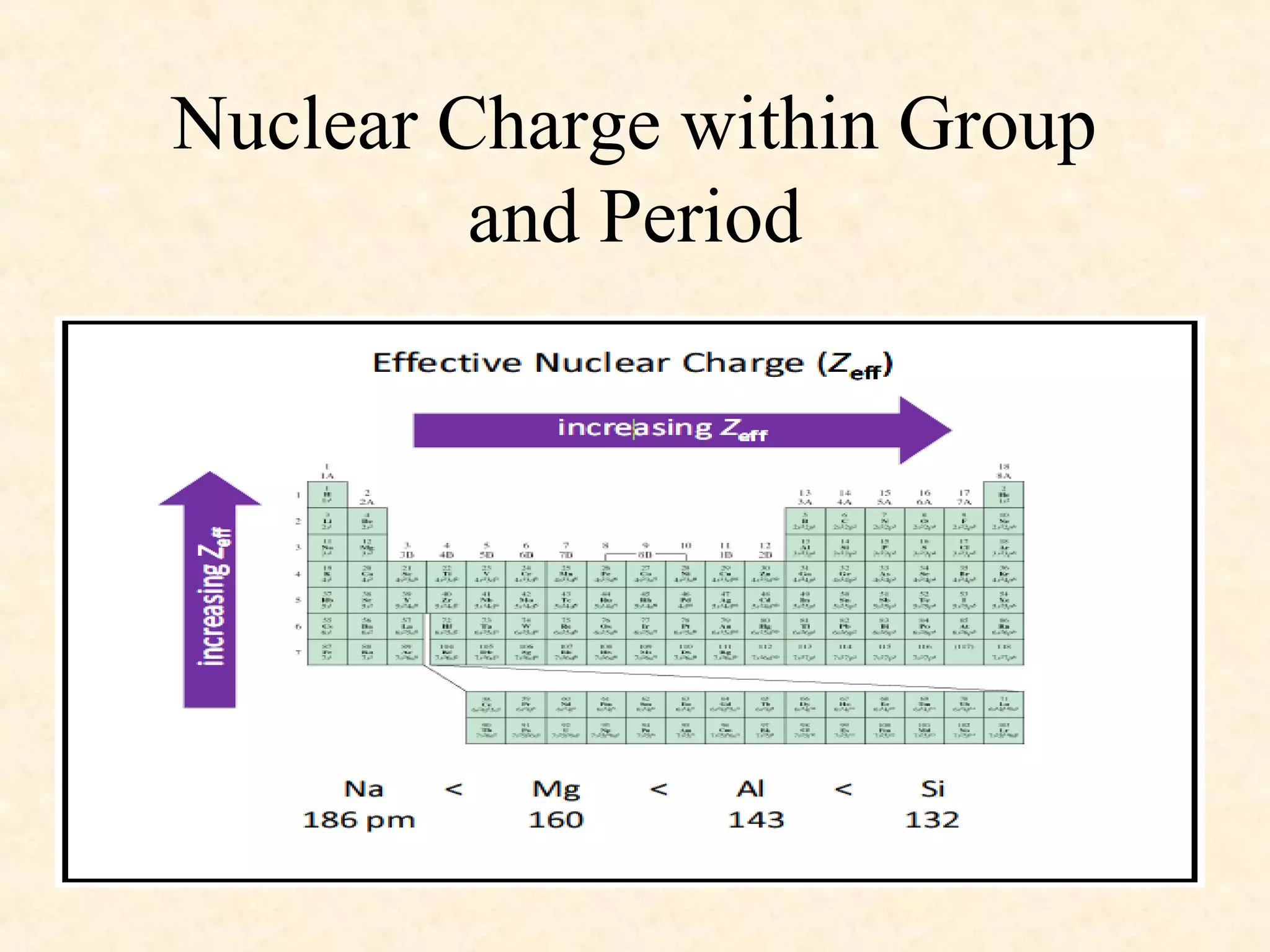
The document discusses several atomic properties including atomic radius, ionic radius, ionization energy, electronegativity, nuclear charge, and shielding effect. It provides trends within the periodic table, noting that atomic radius increases down a group and decreases left to right across a period. Ionic radius also decreases left to right within a period and increases down a group. Ionization energy increases left to right within a period and decreases down a group. Electronegativity increases left to right within a period and decreases down a group. The document also examines nuclear charge and shielding effect.