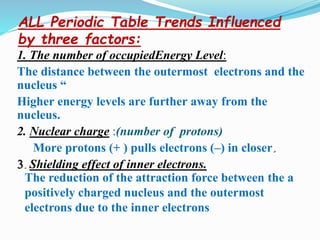
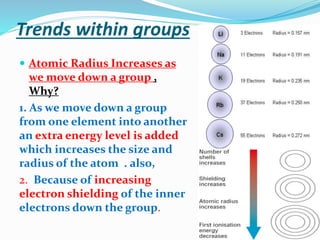
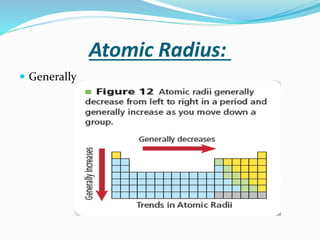
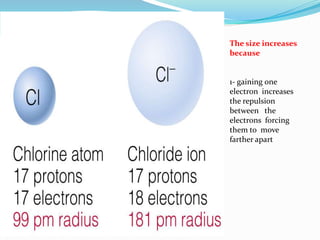
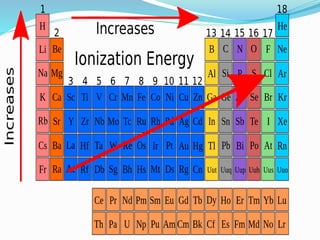
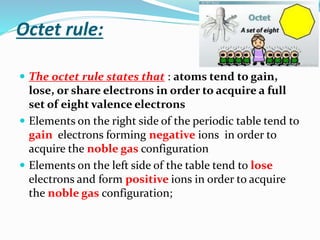
The document discusses the periodic trends of atomic and ionic radii, ionization energy, and electronegativity, emphasizing how these properties change across periods and groups in the periodic table. Key factors include nuclear charge, electron shielding, and the octet rule, which influence atomic behavior and predict chemical interactions. Additionally, it highlights how the size and energy needed to remove electrons varies with electron configuration and the position of elements in the periodic table.