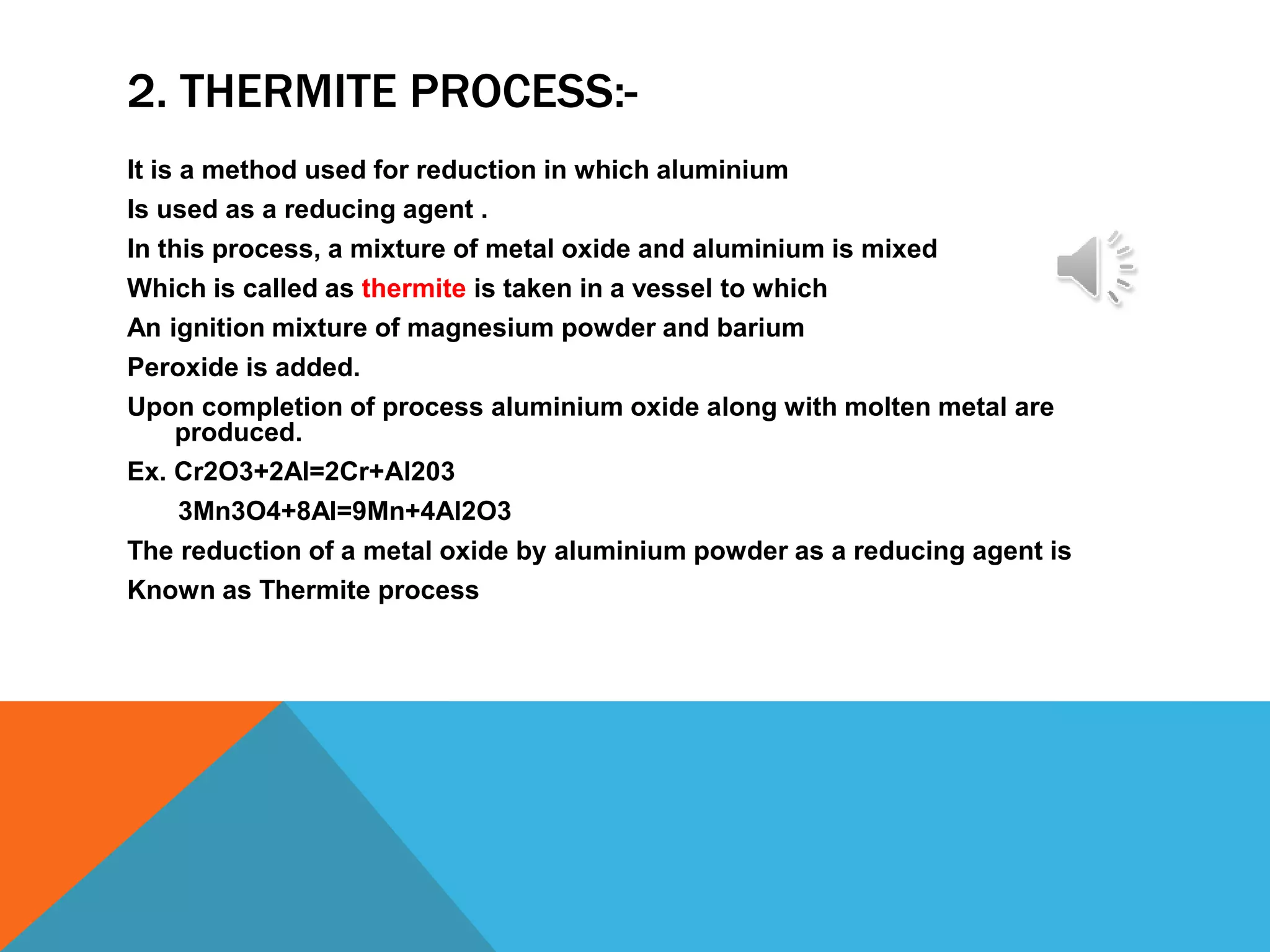
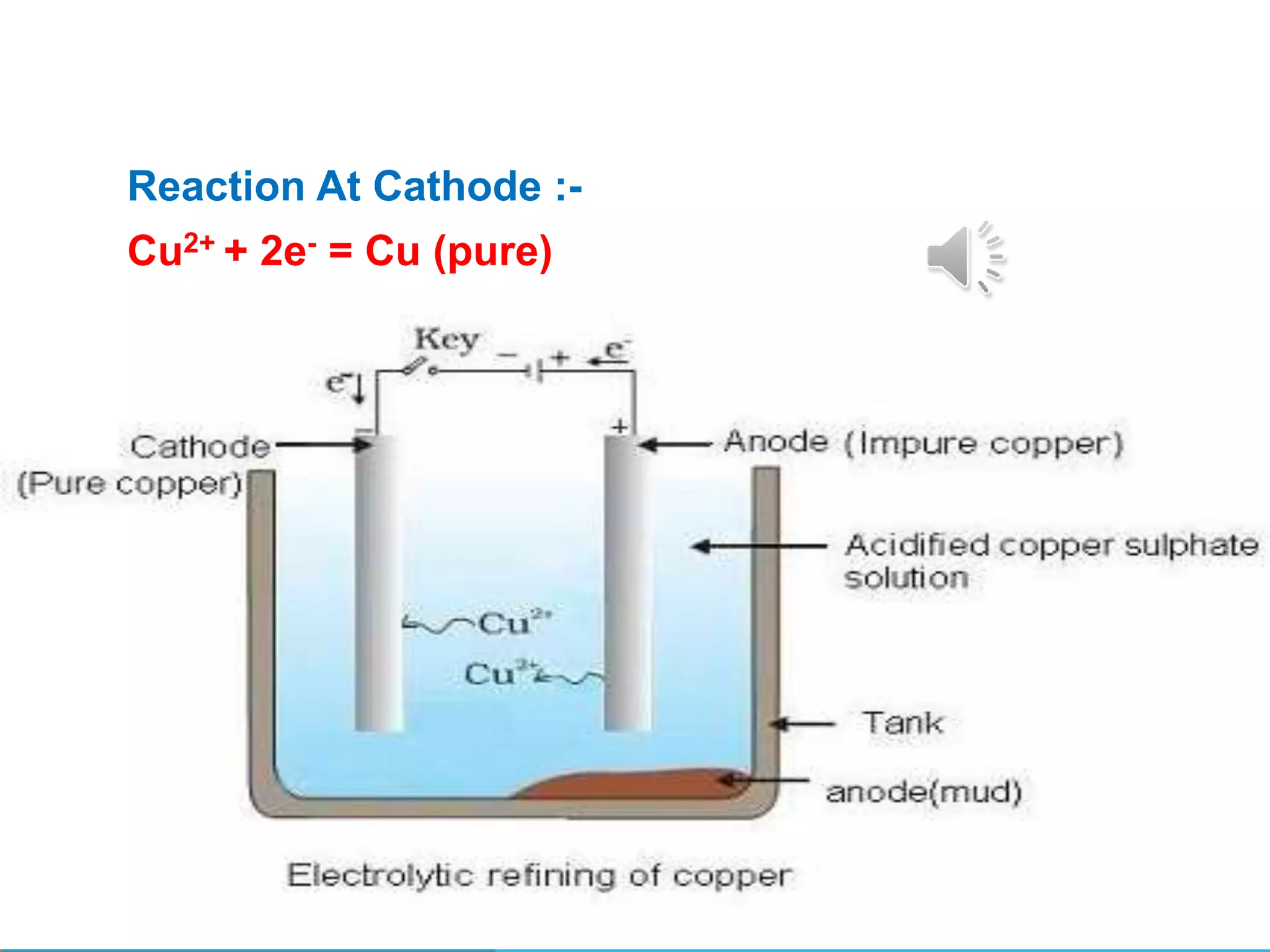
Metallurgy involves extracting metals from their ores in their pure state. Most reactive metals are found combined as sulfates, carbonates, or oxides, while less reactive metals occur naturally in their free metallic state. The process of metallurgy includes mining ores, concentrating the ores to remove impurities, oxidizing and reducing the ores, and refining the reduced metals. Electrolytic refining is used to remove soluble and insoluble impurities from reduced metals to produce pure metals.




![TYPES OF ORES:-
Oxides
Ores
Carbonates Sulphates Sulphides
Haematite
{Fe2O3}
Limestone
[CaCO3]
Espom Salt
[MgSO4.7H2O]
Galena
[Pbs]
Magnetite
[Fe3O4]
Dolomite
[CaCo3.MgCo3]
Gypsum
[Caso4.2H2O]
Cinnabar
[HgS]
Bauxite
{Al2o3.2H2O}
Malachite
[CuCo3.Cu(OH)2
]
Barytes
[BaSO4]
Zinc Blende
[ZnS]
Magnesite
{MgCO3}
Calamine
[ZnCO3]
Anglesite
[PbSO4]
Argentite
[Ag2S]
Zincite{ZnO
}
Siderite[FeCO3] Thernardite[Na2SO4] Iron pyrite[FeS2]
Corundum
{Al2O3}
Azurite
[Cu(OH)2.2CuCO
3
Polyhalite
[K2Ca2MgSO4.2H2O]
Chalcopyrite
[CuFeS2]
Carriterite
{SnO2}](https://image.slidesharecdn.com/metallurgy-201219142025/75/Metallurgy-5-2048.jpg)