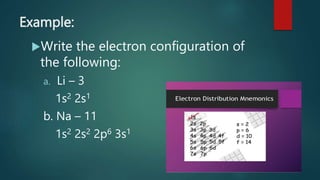
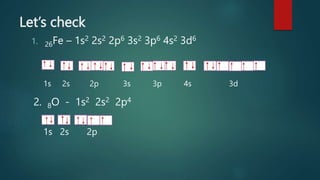
This document discusses electron configuration and orbital diagrams. It defines electron configuration as the arrangement of electrons in atomic orbitals, following standard notation. Orbital diagrams visually represent electron arrangement using labeled circles for orbitals. The document explains the Aufbau principle, Pauli exclusion principle, and Hund's rule for determining electron configuration. It provides examples of writing configurations and drawing diagrams for various elements like Li, Na, N, Al, Cl, Fe, O, and Ca. The document emphasizes that rules are important in electron configurations as they are in other areas of life.