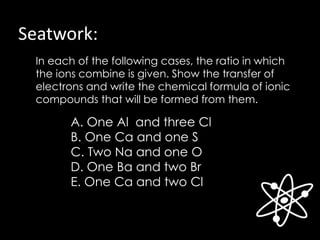
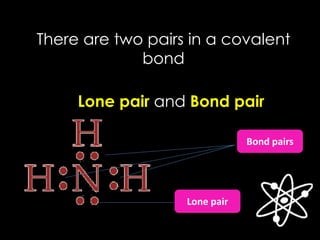
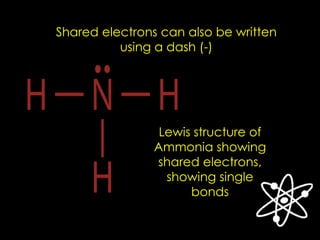
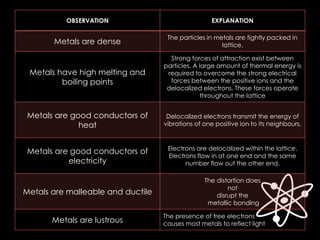
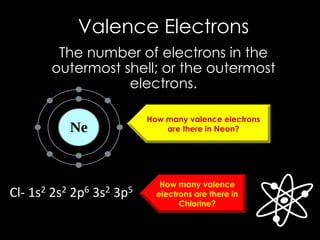
The document discusses different types of chemical bonds, including ionic, covalent, and metallic bonds, along with related concepts such as valence electrons, electronegativity, and ionization energy. It explains how these bonds form through electron transfer or sharing, illustrated with Lewis dot diagrams and the octet rule. Additionally, it highlights the properties of metals and their behavior in metallic bonding.








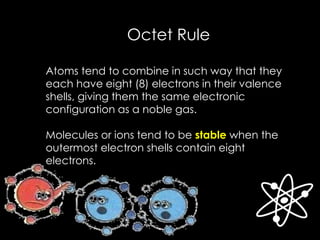






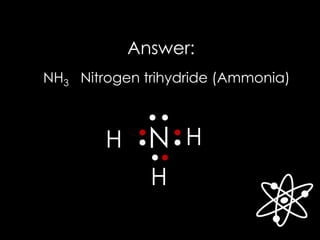
![Answer:
The Lewis structure for Na and O are:
[Na]+
[Na]+
O
X X
X X
X X[ ]
2-
Sodium Oxide (Na2O)](https://image.slidesharecdn.com/chemicalbonding-180917035436/85/Chemical-bonding-UPDATED-21-320.jpg)
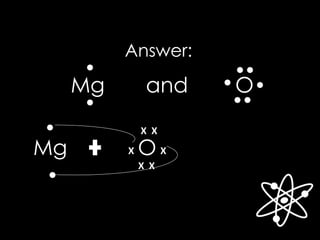
![Answer:
[Mg] O
X X
X X
X X
The Lewis structure for Mg and O are:
[ ]2+ 2-
Magnesium Oxide (MgO)](https://image.slidesharecdn.com/chemicalbonding-180917035436/85/Chemical-bonding-UPDATED-23-320.jpg)