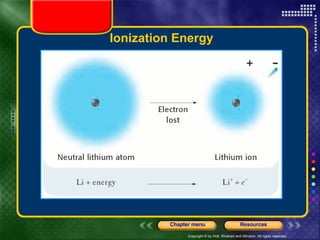
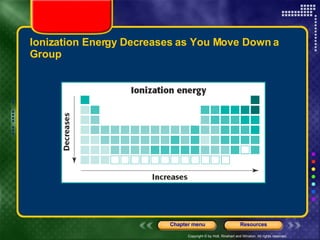
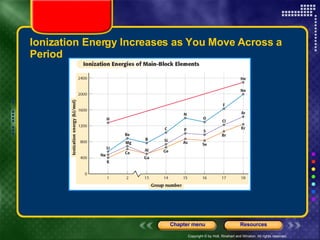
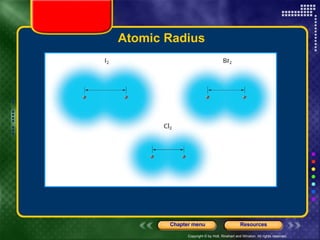
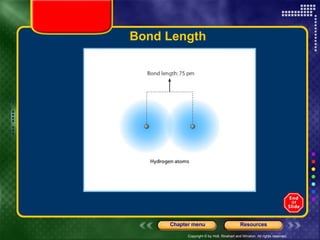
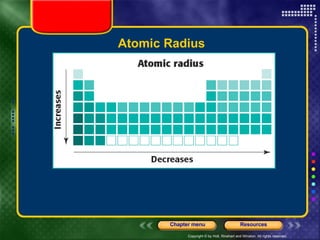
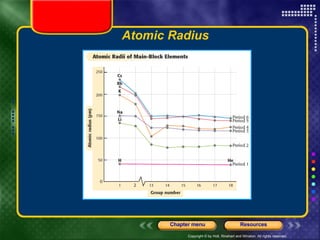
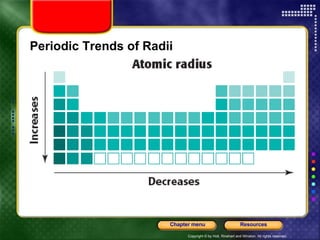
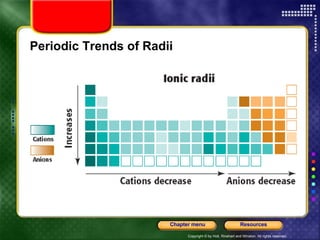
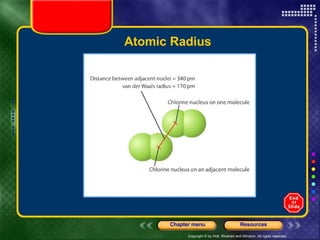
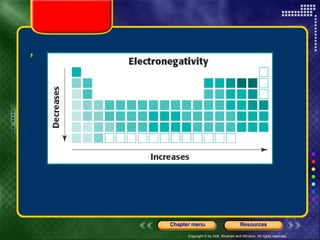
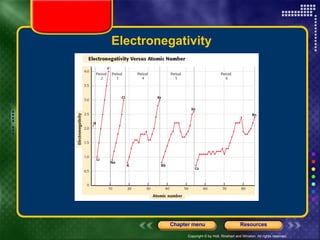
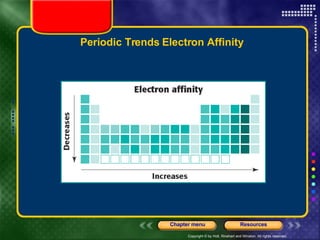
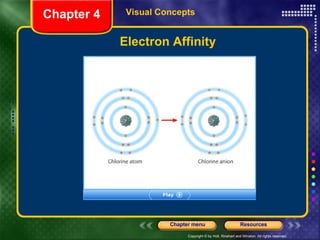
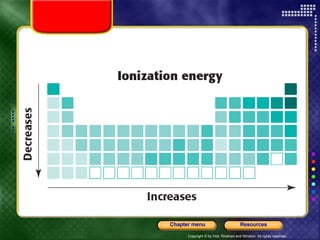
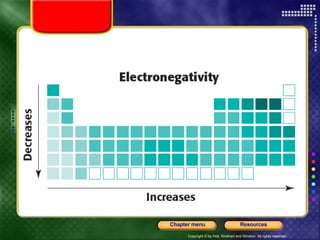
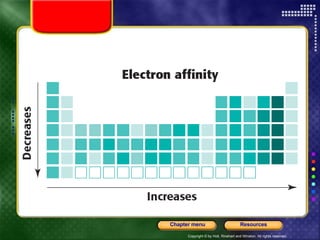
This document discusses periodic trends in properties such as ionization energy, atomic radius, electronegativity, and electron affinity. It explains that these properties generally increase or decrease predictably across periods and down groups on the periodic table due to factors like nuclear charge, electron shielding, and electron configuration. Predictable trends in properties can be understood and used to make inferences about elements based on their positions in the periodic table.