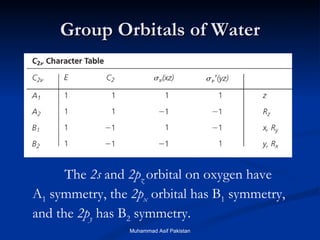
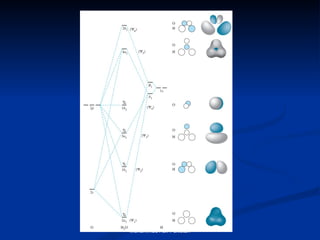
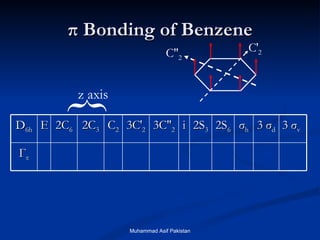
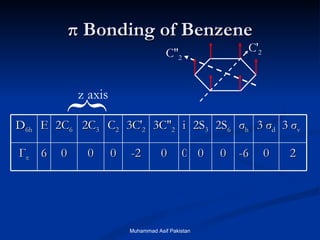
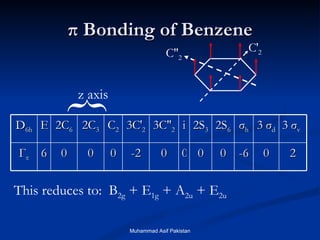
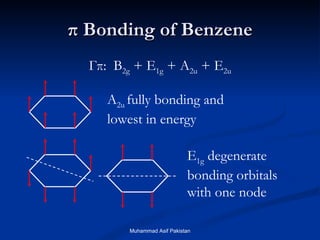
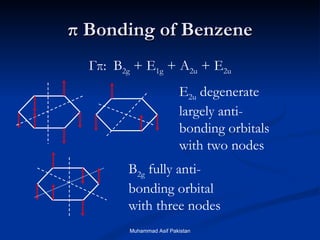
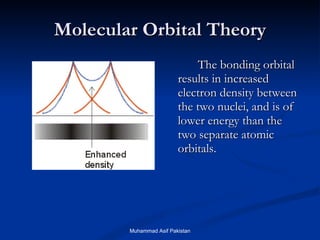
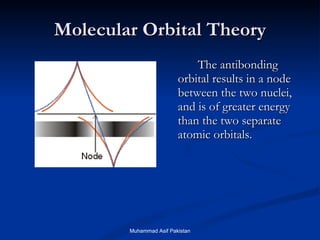
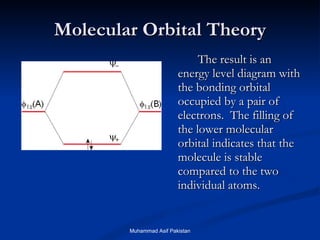
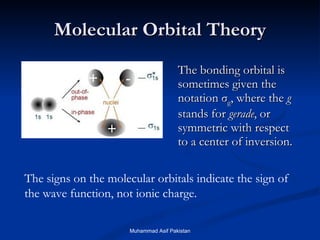
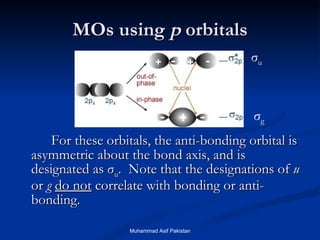
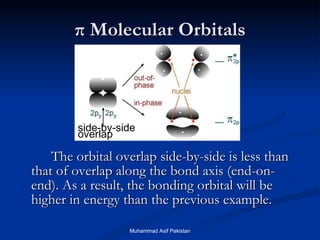
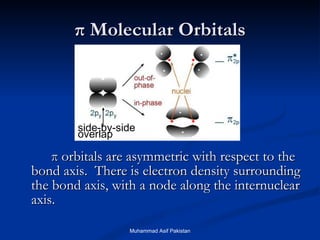
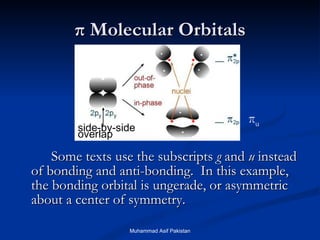
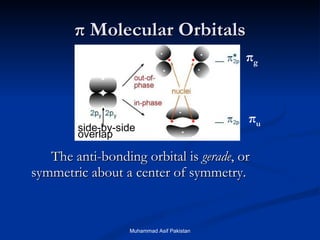
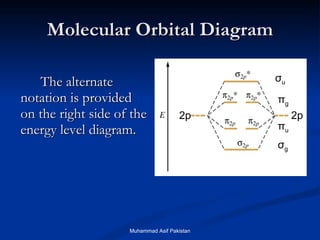
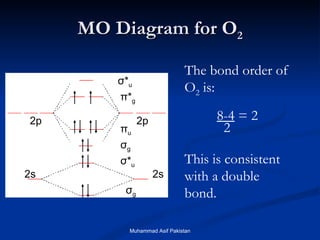
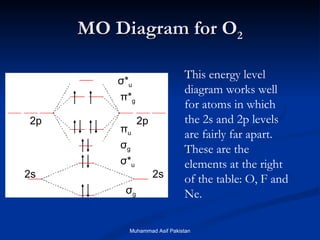
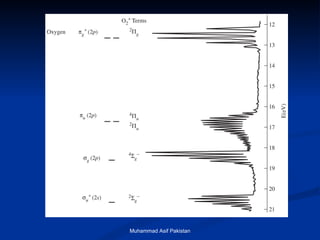
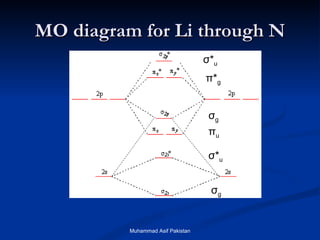
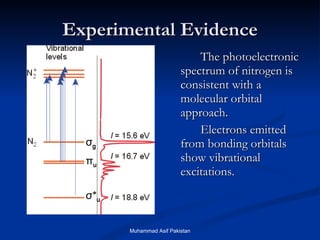
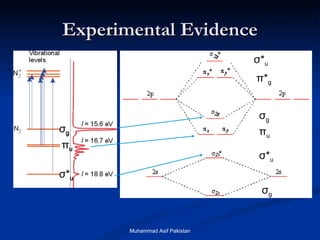
Molecular orbital theory provides an approach to bonding that considers orbitals encompassing the entire molecule rather than just between atoms. Molecular orbitals form from the combination of atomic orbitals, which can combine constructively to form bonding orbitals or destructively to form antibonding orbitals. Bonding orbitals lower the energy and increase stability of the molecule, while antibonding orbitals have higher energy.






![Molecular Orbital Theory Constructively: Ψ ( σ ) or Ψ + = ( 1/√2 ) [ φ (1s a ) + φ (1s b ) ] Destructively: Ψ ( σ *) or Ψ - = ( 1/√2 ) [ φ (1s a ) - φ (1s b ) ] Muhammad Asif Pakistan](https://image.slidesharecdn.com/molecularorbitals-111216142447-phpapp02/85/Molecular-orbitals-8-320.jpg)






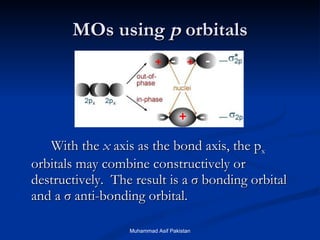
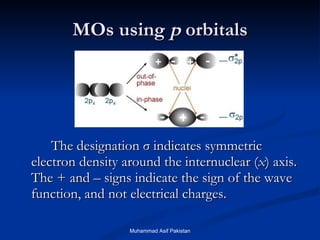
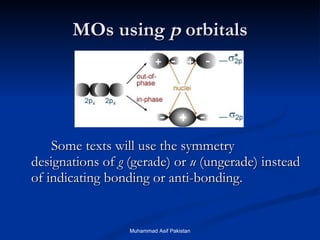
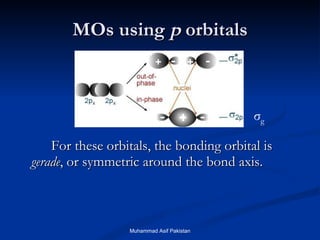

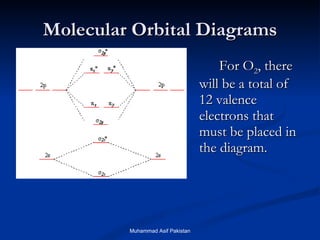
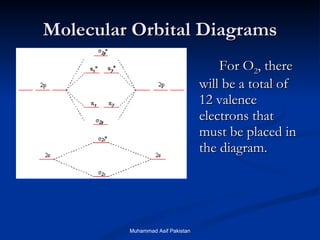
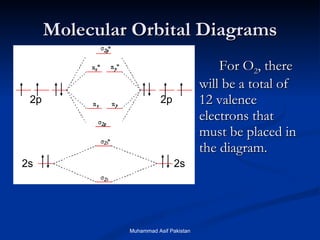
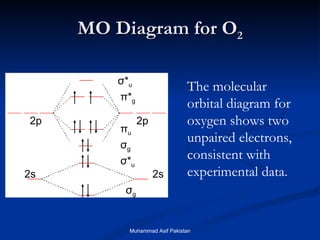




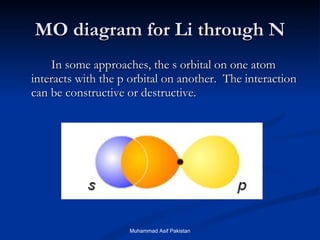




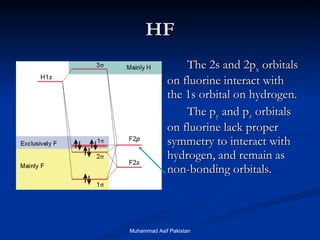
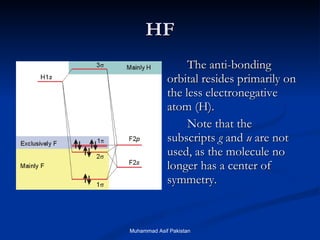
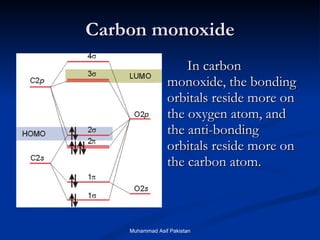
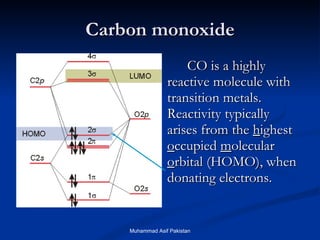
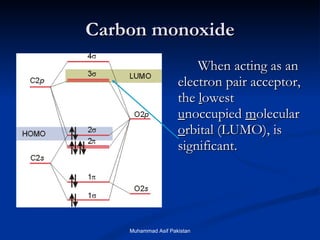
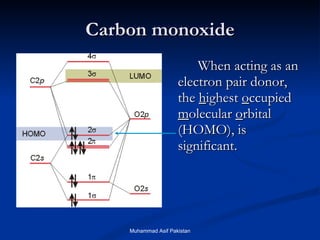
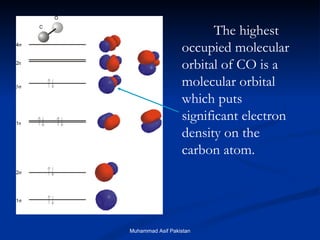
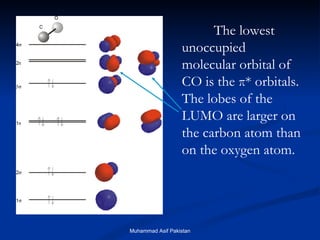
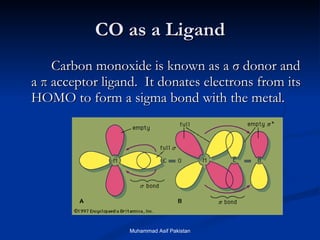
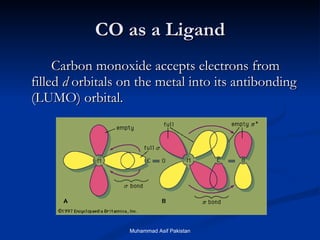
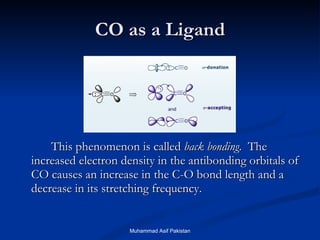
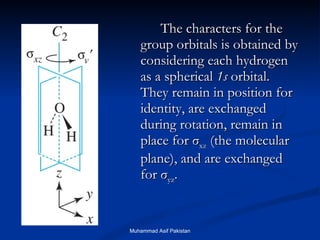
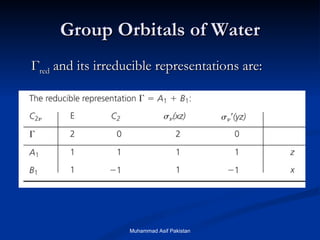
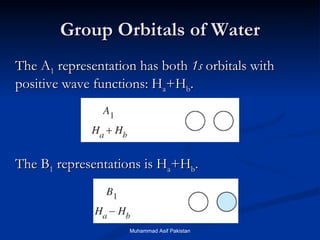
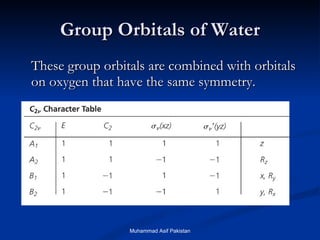
![MOs for Larger Molecules Group theory is usually used to develop molecular orbital diagrams and drawings of more complicated molecules. When a central atom is bonded to several atoms of the same element (H 2 O, BF 3 , or PtCl 4 2- ], group theory can be used to analyze the symmetry of the orbitals of the non-central atoms, and then combine them with the appropriate orbitals of the central atom. Muhammad Asif Pakistan](https://image.slidesharecdn.com/molecularorbitals-111216142447-phpapp02/85/Molecular-orbitals-62-320.jpg)