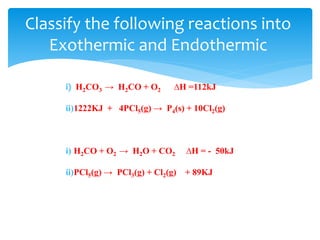
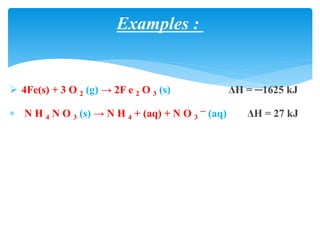
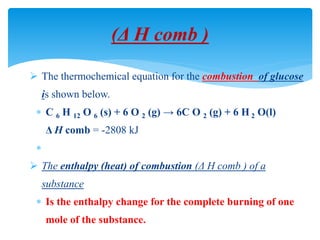
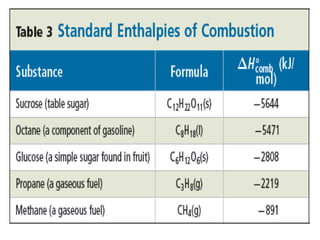
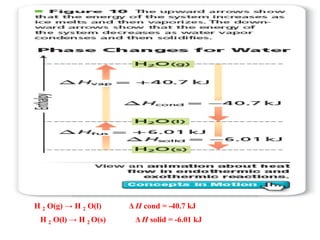
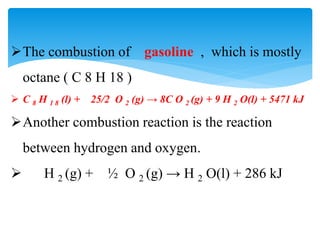
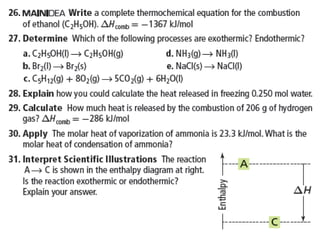
Chapter 15 focuses on thermochemical equations, energy changes in chemical reactions, and the calculation of heat absorbed or released. It explains the classification of reactions as exothermic or endothermic, and details standard enthalpy changes along with examples of combustion and phase changes like vaporization and fusion. Key concepts include the significance of enthalpy changes (δh) in processes and how these changes represent energy absorbed or released during chemical reactions and phase transitions.