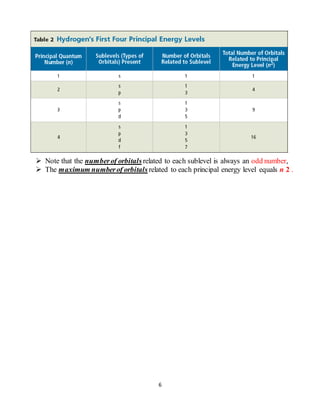
This document provides an overview of Bohr's model of the atom and the development of the quantum mechanical model. It discusses key aspects of Bohr's model including discrete energy levels and allowed electron orbits. It then describes limitations of Bohr's model and the proposal of the quantum mechanical model treating electrons as waves. The quantum mechanical model uses wave functions and orbitals to describe electron location probabilities rather than defined orbits. It introduces the concepts of principal and subenergy levels characterized by quantum numbers that specify allowed electron configurations.