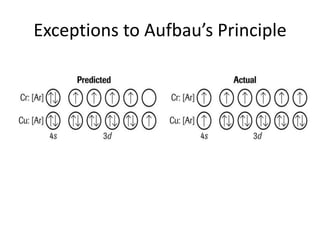
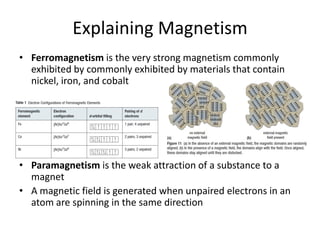
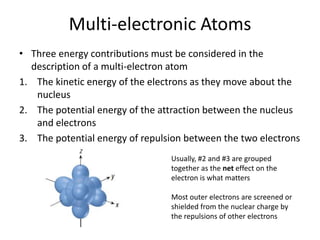
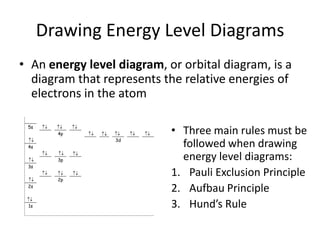
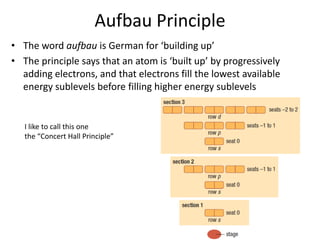
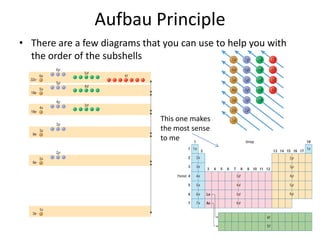
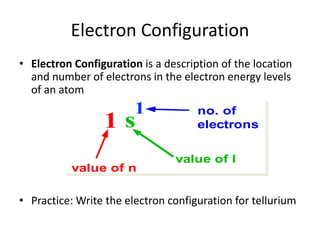
The periodic table arranges elements based on electron configuration in atoms. Elements in the same group have similar valence electron structures and chemical properties. Electrons fill atomic orbitals according to the Aufbau principle and Hund's rule. Valence electrons determine how elements bond and react. Ion charges form when atoms gain or lose valence electrons to achieve stable full shells like noble gases. Magnetism results from aligned spins of unpaired electrons.


![Ways of Expressing Electron
Configurations
• Full configuration
– Complete ordered placement starting with 1s2
• 1s2 2s2 2p6 3s2 3p6 4s2 3d4
• 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
• Condensed configuration
– Completed noble gas configuration in brackets followed by
detail for unfilled valence shell
• [Ar]4s2 3d4
• [Ar] 4s2 3d10 4p2
• Orbital diagrams for outer valence shell
– Labeled picture of outer valence shell](https://image.slidesharecdn.com/35atomicstructureandtheperiodictable-150410004839-conversion-gate01/85/35-atomicstructureandtheperiodictable-10-320.jpg)