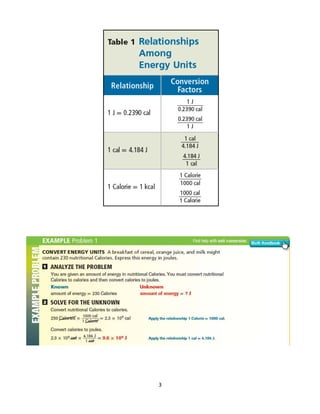
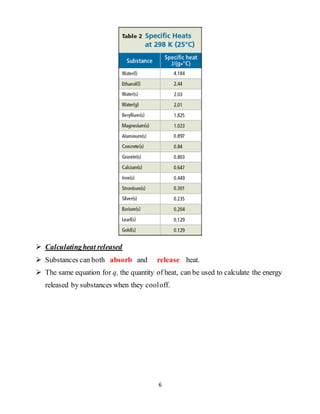
This document provides information about energy and chemical changes from a chemistry textbook. It defines energy as the ability to do work or produce heat, and discusses the two main forms of energy as potential and kinetic. Potential energy is due to the position or composition of an object, while kinetic energy is energy of motion. It also introduces the law of conservation of energy, which states that energy cannot be created or destroyed, only changed from one form to another. The document provides examples of potential, kinetic, chemical, and heat energy. It defines heat as energy transferred between objects at different temperatures and discusses units used to measure heat. The specific heat of a substance, or amount of heat needed to change its temperature, is also explained. Examples of