This document discusses key concepts in chemical kinetics including:
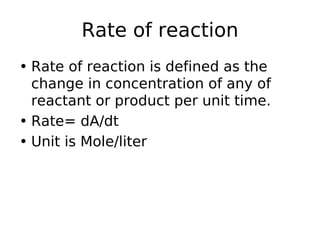
- Rate of reaction is defined as the change in concentration of reactants or products per unit time. Rate laws describe how the rate of reaction depends on reactant concentrations.
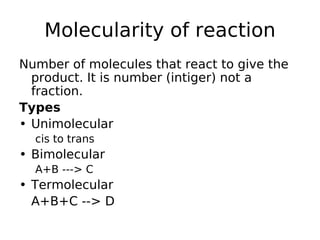
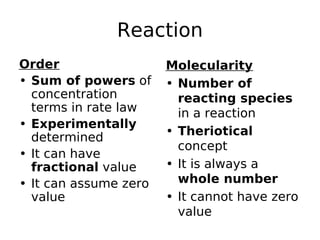
- Order of reaction refers to the sum of powers of concentrations in the rate law. Molecularity is the actual number of reacting species.
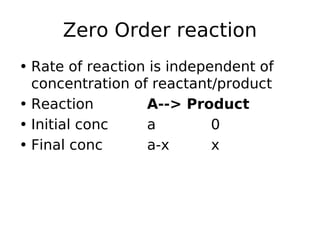
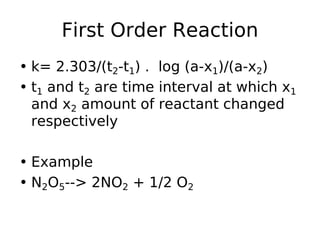
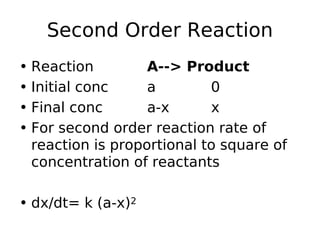
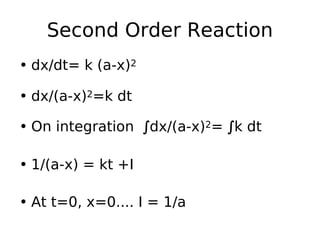
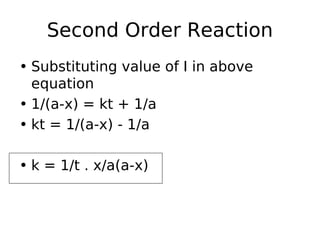
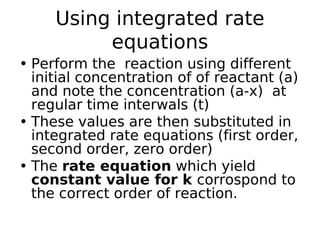
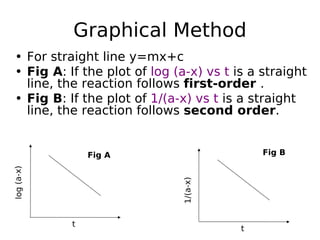
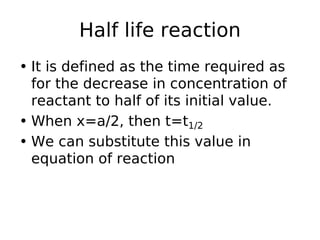
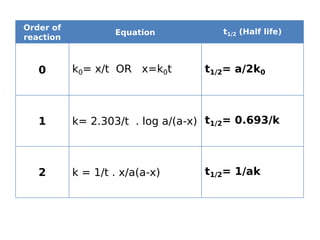
- Reaction orders include zero order (independent of concentration), first order, and second order reactions. Integrated rate equations relate concentration changes to rate constants for each order.
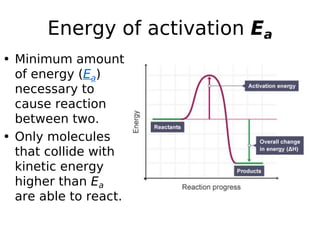
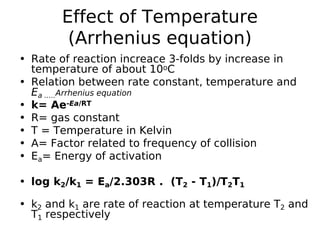
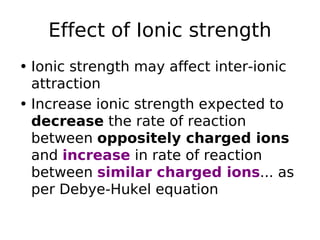
- Factors like temperature, solvent, ionic strength, and catalysis influence reaction rates as described by theories like collision theory and Arrhenius equation. Determining

![Rate Law
• The rate of reaction is directly
proportional to the reactant
concentration, each concentration
being raised to some power.
• 2A + B --> Product
• Rate =k[A]m[B]n
(k = specific rate constant)
The Equation shows how rate is related to concentration](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-3-320.jpg)
![Order of reaction
• Order of reaction is defined as sum of
powers of concentration in rate law.
• Rate =k[A]m[B]n
• Order of reaction in above case is
(m+n)
• It is the number of concentration
terms on which the rate of reaction
depends.](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-4-320.jpg)
![Zero Order reaction
• Rate of reaction = -d[A]/dt= k0[A]0
• dx/dt= k0 (a-x)0=k0
• x=k0t
• k0 is rate constant (or specific rate
constant) of zero order reaction
• Rate constant is the rate of reaction
at all concentrations x/t](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-8-320.jpg)


![Pseudo order reaction
• Experimental order of reaction which
is not actual is known as pseudo
order
• Reaction A+B --> Product
• If B is in excesses, its concentration
will practically constant and only
concentration of A will affect rate of
reaction hence rate law will be..
• Rate = k' [A]...](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-15-320.jpg)

![Units of rate constant
• Units of rate constant for different
order reaction are different
• For Zero order
• k= d[A]/dt
• k= mol/lit . 1/time
• k= mol lit-1 time-1](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-17-320.jpg)
![Units of rate constant
• For first order
• k= 2.303/t . log a/(a-x)
• k= 2.303/t . log [A]0/[A]t
• k= 1/time
• k= time-1](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-18-320.jpg)
![Units of rate constant
• For second order reaction
• k= 1/t . x/a(a-x)
• k= 1/t . x/[A]0([A]0-x)
• k= 1/time . concentration/concentration2
• k= 1/time . 1/concentration
• k= 1/time . 1/(mol/lit)
• k= mol-1 lit time-1](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-19-320.jpg)

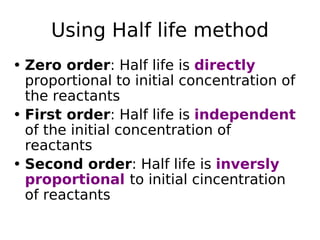
![Using Half life method
• Seperate experiments should be
performed using different initial
concentration
• Half life for nth order reaction is
• t1/2= 1/ [A] n-1](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-24-320.jpg)




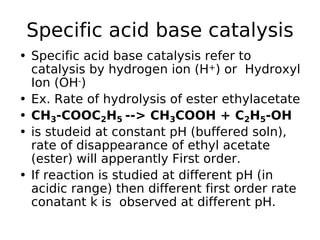
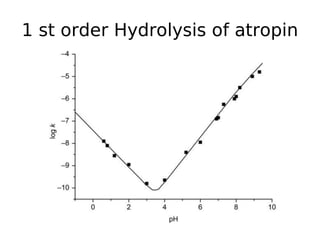
![Specific acid base catalysis
• Observed rate depend on
concentration of ester and [H+],
therefore it is actually second order
reaction.
• Observed rate constant (kobs) is
proportional to [H+]
• kobs = kacid [H+]
• logkobs = log kacid + log [H+]
• logkobs = log kacid - pH](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-35-320.jpg)
![Specific acid base catalysis
• Log kobs = log kacid - pH
• It suggest kobs vs pH is straight line
with slop -1 and y-intercept log kacid
• If we study same reaction in alkaline
pH then we observe different rate
constant at different pH
• log kobs = log kbase + log [OH-]
• kobs = kacid[H+] + kbase [OH-]](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-36-320.jpg)
![General acid base catalysis
• Acid or base catalysis is not
restricted to effect of [H+] or [OH-]
• Undissociated acid and base can
often produce catalytic effect
• Metal can also serve as catalyst
• Ex. Mutarotation of glucose in
acetate buffer is catalysed by [H+],
[OH-], Acetate ion [CH3COO-],
undissociated acid [CH3COOH]
undissociated acetic acid.](https://image.slidesharecdn.com/chemicalkineticsanddrugstability-180123165311/85/Chemical-kinetics-38-320.jpg)




