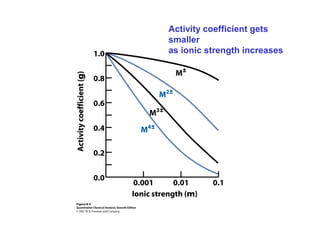
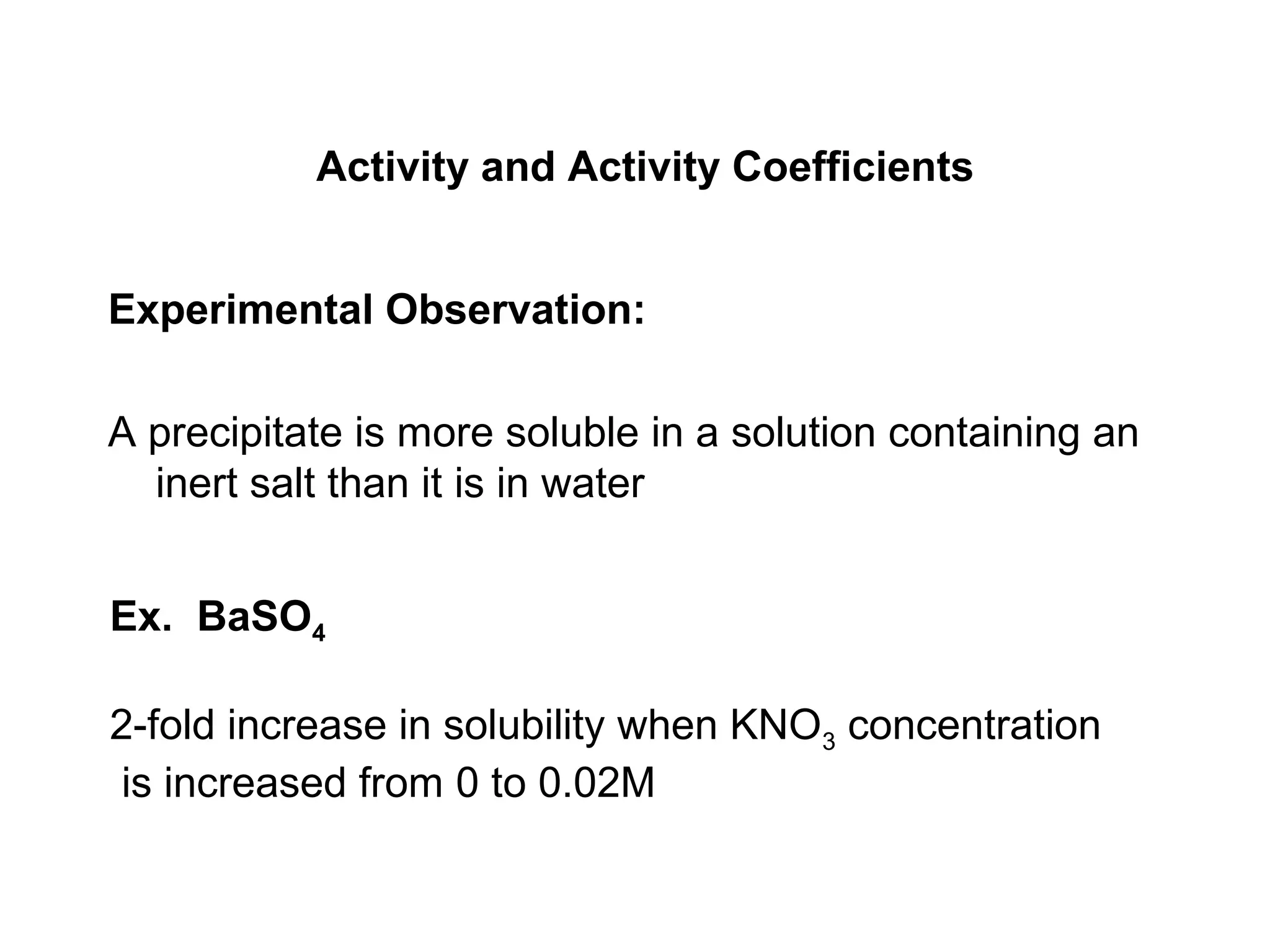
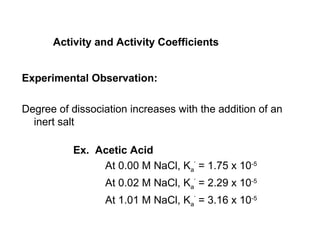
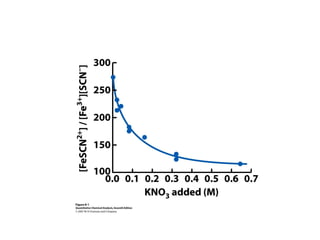
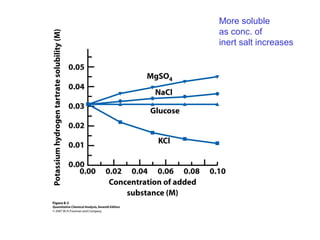
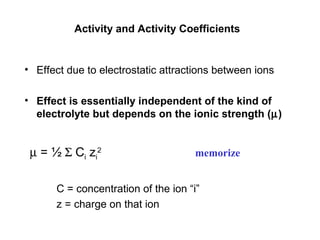
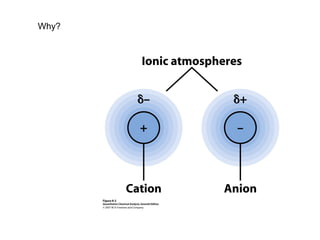
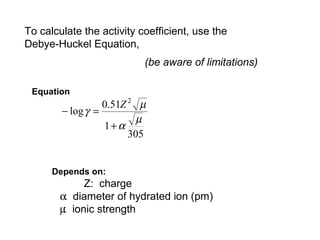
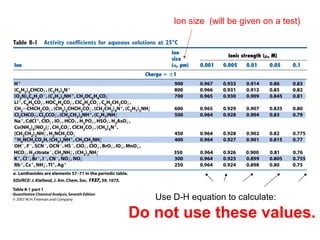
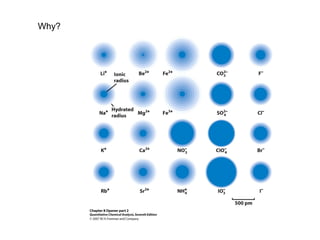
This document discusses how activity coefficients can explain the effect of inert salts on solubility and acid dissociation constants. It provides examples showing that a precipitate is more soluble and a weak acid dissociates more when the ionic strength is increased by adding an inert salt. This is because the activity coefficients of the ions are less than 1 and decrease with increasing ionic strength, making the activities higher than concentrations. The Debye-Huckel equation can be used to calculate activity coefficients based on ionic charge and strength.
![Activity and Activity Coefficients
aA + bB = cC + dD
K = [C]c
[D]d
/ [A]a
[B]b
Does not predict any effect on the ionic strength](https://image.slidesharecdn.com/chapter7activity-140614111320-phpapp02/85/Chapter-7-activity-6-320.jpg)
![Activity and Activity Coefficients
activity Activity coefficient
Ac = [C] γC
K = AC
c
AD
d
/ AB
b
AA
a
K = γC
c
[C]c
γD
d
[D]d
/ γB
b
[B]b
γA
a
[A]a](https://image.slidesharecdn.com/chapter7activity-140614111320-phpapp02/85/Chapter-7-activity-8-320.jpg)
![Activity and Activity Coefficients
Examples:
Sparingly soluble salt
BaSO4(s) = Ba2+
+ SO4
2-
Ksp = ABaASO4 = [Ba][SO4] γBaγSO4
pH
pH = - log AH+ = -log [H+
] γH+](https://image.slidesharecdn.com/chapter7activity-140614111320-phpapp02/85/Chapter-7-activity-9-320.jpg)
![Activity and Activity Coefficients
Weak Acid
HA + H2O = A- + H30+
Ka = ([A-][H3O+] / [HA]) (γA-γH3O / γHA)](https://image.slidesharecdn.com/chapter7activity-140614111320-phpapp02/85/Chapter-7-activity-10-320.jpg)
![Activity and Activity Coefficients
Properties of Activity Coefficients:
• Measure of the “effectiveness” with which that
species influences an equilibrium
µ 0, γx 1, ax [X], K’
K
• Dilute solutions, γx is independent of the nature of
electrolyte and dependent on µ
• For a given µ, γx departs farther from unity as
the charge increases](https://image.slidesharecdn.com/chapter7activity-140614111320-phpapp02/85/Chapter-7-activity-14-320.jpg)