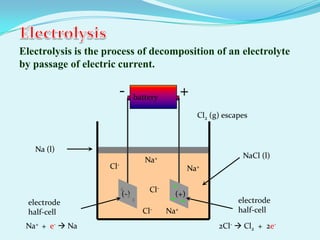
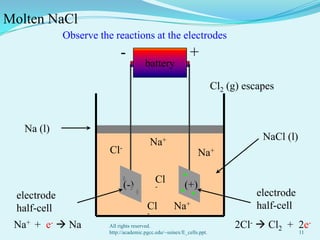
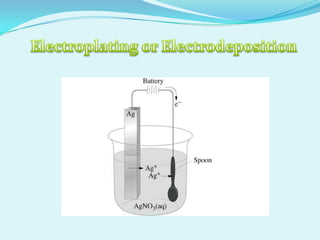
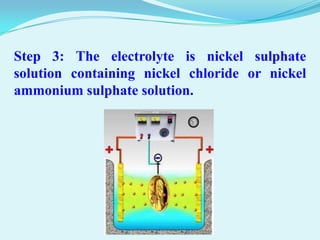
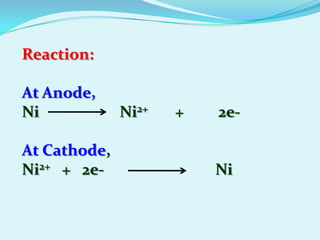
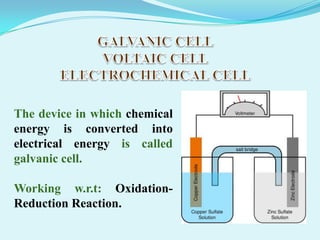
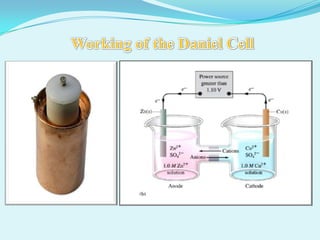
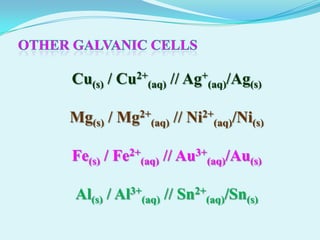
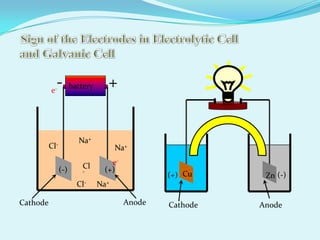
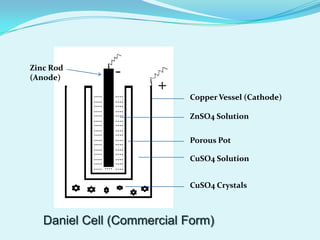
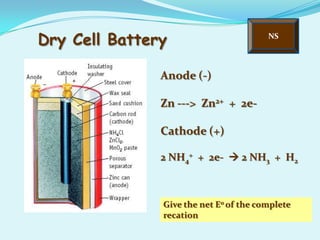
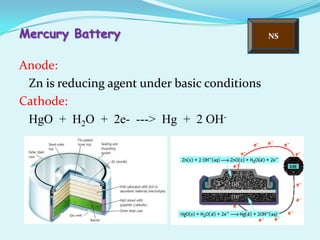
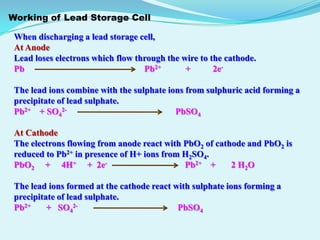
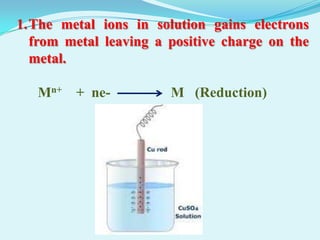
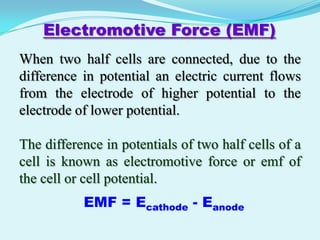
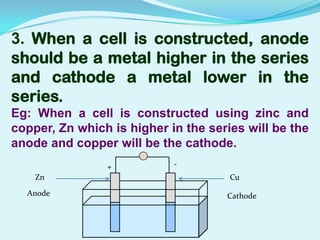
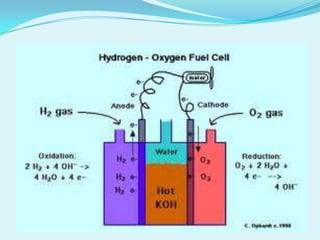
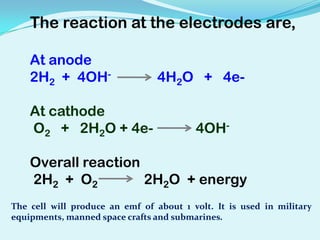
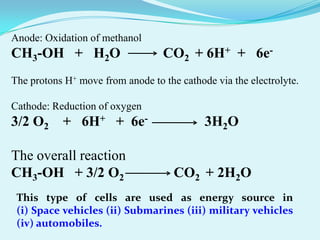
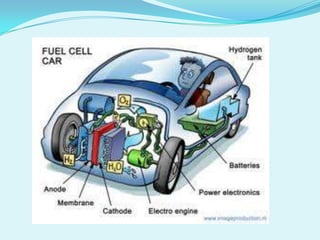
The document discusses different types of electrochemical cells including primary cells that produce electricity from non-reversible chemical reactions and secondary cells that can be recharged by passing electricity in the opposite direction of the spontaneous reaction. Examples of primary cells discussed include Daniel, mercury, dry, and alkaline cells, while examples of secondary cells include lead-acid, nickel-cadmium, nickel-metal hydride, and lithium-ion batteries. The working and reactions of common cells like lead-acid, alkaline, and dry cells are also explained.