The document discusses chemical kinetics, focusing on the rate and order of reactions, describing rate laws based on experimentally derived equations relating reaction rates to reactant concentrations. It explains the determination of rate equations through initial rate and graphical methods and provides examples to illustrate the concepts, including various order reactions. Overall, it serves as a comprehensive guide for understanding and calculating reaction rates in chemical processes.


![3
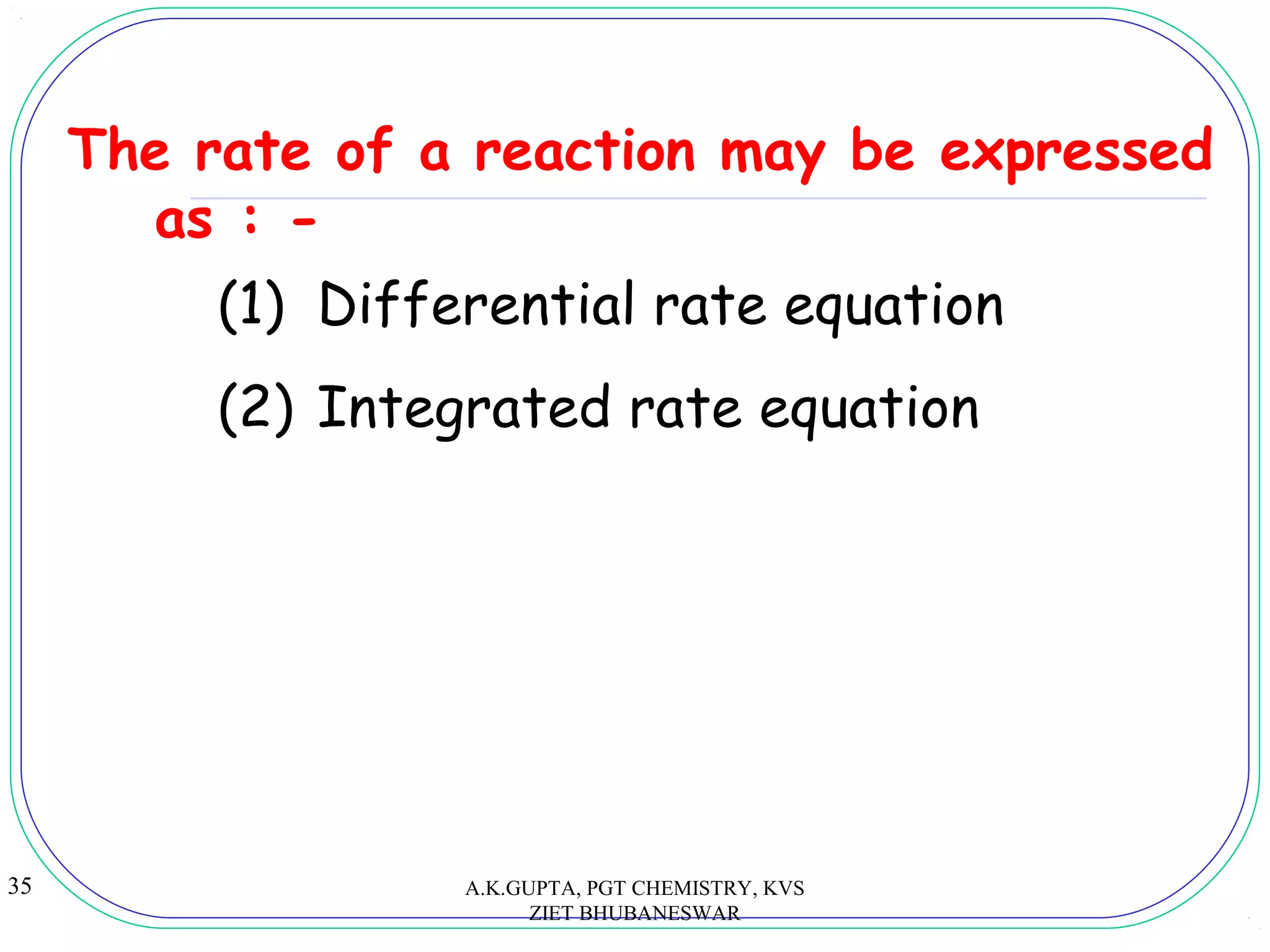
For the reaction aA + bB → cC + dD
Rate = k[A]n
[B]m
Rate law or rate equation:
Experimentally derived algebraic
equation which relates the rate of
reaction with the concentration of
the reactants
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-3-2048.jpg)
![4
For the reaction aA + bB → cC + dD
Rate = k[A]n
[B]m
where n and m are the orders of reaction
with respect to A and B
n and m can be ± integers or fractional
n + m is the overall order of reaction.
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-4-2048.jpg)
![5
For the reaction aA + bB → cC + dD
Rate = k[A]n
[B]m
For multi-step reactions, n & y have no direct
relation to the stoichiometric coefficients and
can ONLY be determined experimentally.
For single-step reactions (elementary
reactions),
n = a and m= b
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-5-2048.jpg)
![6
For the reaction aA + bB → cC + dD
Rate = k[A]n
[B]m
n = 0 → zero order w.r.t. A
n = 1 → first order w.r.t. A
n = 2 → second order w.r.t. A
m = 0 → zero order w.r.t. B
m = 1 → first order w.r.t. B
m = 2 → second order w.r.t. B
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-6-2048.jpg)
![7
For the reaction aA + bB → cC + dD
Rate = k[B]2
Describe the reaction with the following rate law.
The reaction is zero order w.r.t. A and
second order w.r.t. B.
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-7-2048.jpg)
![8
Rate = k[A]n
[B]m
Where k is the rate constant
(specific rate) of the reaction
For the reaction aA + bB → cC + dD
• Temperature-dependent
• Can only be determined from experiments
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
k is](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-8-2048.jpg)
![9
Rate = k[A]n
[B]m
units of k : -
mol L−1
s−1
/(mol L−1
)n+m
or,
mol L−1
min−1
/(mol L−1
)n+m
For the reaction aA + bB → cC + dD
m1n1
11
mn
)L(mol)L(mol
sLmol
[B][A]
rate
k −−
−−
==
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-9-2048.jpg)
![10
Rate = k[A]0
[B]0
units of k
= mol L−1
s−1
/(mol L−1
)0+0
= mol L−1
s−1
= units of rate
For the reaction aA + bB → cC + dD
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-10-2048.jpg)
![11
Rate = k[A][B]0
units of k
= mol L−1
s−1
/(mol L−1
)1+0
= s−1
For the reaction aA + bB → cC + dD
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-11-2048.jpg)
![12
Rate = k[A][B]
units of k
= mol L−1
s−1
/(mol L−1
)1+1
= mol−1
L1
s−1
For the reaction aA + bB → cC + dD
The overall order of reaction can be
deduced from the units of k
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-12-2048.jpg)
![13
Rate = k[A]n
[B]m
[C]p
…
For the reaction
aA + bB + cC + … → products
units of k : -
mol L−1
s−1
/(mol L−1
)n+m+p+…
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-13-2048.jpg)
![14
Determination of rate equations
To determine a rate equation is to find n, m, p,
z,…
Rate = k[A]n
[B]m
[C]p
…
Two approaches : -
1. Initial rate method
2. Graphical method
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-14-2048.jpg)

![16
5Cl−
(aq) + ClO3
−
(aq) + 6H+
(aq) → 3Cl2(aq) + 3H2O(l)
Expt
[Cl−
(aq)]
(mol L−1
)
[ClO3
−
(aq)]
(mol L−1
)
[H+
(aq)]
(mol L−1
)
Initial rate
(mol L−1
s−1
)
1 0.15 0.08 0.20 1.0×10−5
2 0.15 0.08 0.40 4.0×10−5
3 0.15 0.16 0.40 8.0×10−5
4 0.30 0.08 0.20 2.0×10−5
Suppose the rate law for the reaction is
rate = k[Cl−
(aq)]n
[ClO3
−
(aq)]m
[H+
(aq)]p
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-16-2048.jpg)
![17
Expt
[Cl−
(aq)]
(mol L−1
)
[ClO3
−
(aq)]
(mol L−1
)
[H+
(aq)]
(mol L−1
)
Initial rate
(mol L−1
s−1
)
1 0.15 0.08 0.20 1.0×10−5
2 0.15 0.08 0.40 4.0×10−5
3 0.15 0.16 0.40 8.0×10−5
4 0.30 0.08 0.20 2.0×10−5
pmn
pmn
5
5
(0.20)(0.08)(0.15)
(0.40)(0.08)(0.15)
101.0
104.0
=
×
×
−
−
From experiments 1 and 2,
4 = 2p
⇒ p = 2
= 2p
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-17-2048.jpg)
![18
Expt
[Cl−
(aq)]
(mol L−1
)
[ClO3
−
(aq)]
(mol L−1
)
[H+
(aq)]
(mol L−1
)
Initial rate
(mol L−1
s−1
)
1 0.15 0.08 0.20 1.0×10−5
2 0.15 0.08 0.40 4.0×10−5
3 0.15 0.16 0.40 8.0×10−5
4 0.30 0.08 0.20 2.0×10−5
pmn
pmn
5
5
(0.40)(0.08)(0.15)
(0.40)(0.16)(0.15)
104.0
108.0
=
×
×
−
−
From experiments 2 and 3,
2 = 2m
⇒ m = 1
= 2m
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-18-2048.jpg)
![19
Expt
[Cl−
(aq)]
(mol L−1
)
[ClO3
−
(aq)]
(mol L−1
)
[H+
(aq)]
(mol L−1
)
Initial rate
(mol L−1
s−1
)
1 0.15 0.08 0.20 1.0×10−5
2 0.15 0.08 0.40 4.0×10−5
3 0.15 0.16 0.40 8.0×10−5
4 0.30 0.08 0.20 2.0×10−5
pmn
pmn
5
5
(0.20)(0.08)(0.15)
(0.20)(0.08)(0.30)
101.0
102.0
=
×
×
−
−
From experiments 1 and 4,
2 = 2n
⇒ n = 1
= 2n
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-19-2048.jpg)
![20
rate = k[Cl−
(aq)][ClO3
−
(aq)][H+
(aq)]2
Expt
[Cl−
(aq)]
(mol L−1
)
[ClO3
−
(aq)]
(mol L−1
)
[H+
(aq)]
(mol L−1
)
Initial rate
(mol L−1
s−1
)
1 0.15 0.08 0.20 1.0×10−5
2 0.15 0.08 0.40 4.0×10−5
3 0.15 0.16 0.40 8.0×10−5
4 0.30 0.08 0.20 2.0×10−5
From experiment 1,
1.0×10−5
= k(0.15)(0.08)(0.20)2
k = 0.02 mol−3
L3
s−1
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-20-2048.jpg)
![21
rate = k[Cl−
(aq)][ClO3
−
(aq)][H+
(aq)]2
Expt
[Cl−
(aq)]
(mol L−1
)
[ClO3
−
(aq)]
(mol L−1
)
[H+
(aq)]
(mol L−1
)
Initial rate
(mol L−1
s−1
)
1 0.15 0.08 0.20 1.0×10−5
2 0.15 0.08 0.40 4.0×10−5
3 0.15 0.16 0.40 8.0×10−5
4 0.30 0.08 0.20 2.0×10−5
From experiment 2,
4.0×10−5
= k(0.15)(0.08)(0.40)2
k = 0.02 mol−3
L3
s−1
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-21-2048.jpg)
![22
Question 2C + 3D + E → P + 2Q
Exp.
[C]
( mol L−1
)
[D]
( mol L−1
)
[E]
( mol L−1
)
Initial rate
( mol L−1
s-1
)
1 0.10 0.10 0.10 3.0×10−3
2 0.20 0.10 0.10 2.4×10−2
3 0.10 0.20 0.10 3.0×10−3
4 0.10 0.10 0.30 2.7×10−2
(a) rate = k[C]n
[D]m
[E]p
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-22-2048.jpg)
![23
Exp.
[C]
( mol L−1
)
[D]
( mol L−1
)
[E]
( mol L−1
)
Initial rate
( mol L−1
s-1
)
1 0.10 0.10 0.10 3.0×10−3
2 0.20 0.10 0.10 2.4×10−2
3 0.10 0.20 0.10 3.0×10−3
4 0.10 0.10 0.30 2.7×10−2
(a) rate = k[C]n
[D]m
[E]p
pmn
pmn
3
-2
(0.10)(0.10)(0.10)
(0.10)(0.10)(0.20)
103.0
102.4
=
×
×
−
From experiments 1 and 2,
8 = 2n
⇒ n = 3
= 2n
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-23-2048.jpg)
![24
Exp.
[C]
( mol L−1
)
[D]
( mol L−1
)
[E]
( mol L−1
)
Initial rate
( mol L−1
s-1
)
1 0.10 0.10 0.10 3.0×10−3
2 0.20 0.10 0.10 2.4×10−2
3 0.10 0.20 0.10 3.0×10−3
4 0.10 0.10 0.30 2.7×10−2
(a) rate = k[C]n
[D]m
[E]p
pmn
pmn
3
-3
(0.10)(0.10)(0.10)
(0.10)(0.20)(0.10)
103.0
103.0
=
×
×
−
From experiments 1 and 3,
1 = 2m
⇒ m = 0
= 2m
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-24-2048.jpg)
![25
Exp.
[C]
( mol L−1
)
[D]
( mol L−1
)
[E]
( mol L−1
)
Initial rate
( mol L−1
s-1
)
1 0.10 0.10 0.10 3.0×10−3
2 0.20 0.10 0.10 2.4×10−2
3 0.10 0.20 0.10 3.0×10−3
4 0.10 0.10 0.30 2.7×10−2
(a) rate = k[C]x
[D]y
[E]z
pmn
pmn
3
-2
(0.10)(0.10)(0.10)
(0.30)(0.10)(0.10)
103.0
102.7
=
×
×
−
From experiments 1 and 4,
9 = 3p ⇒ p = 2
= 3p
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-25-2048.jpg)
![26
Exp.
[C]
( mol L−1
)
[D]
( mol L−1
)
[E]
( mol L−1
)
Initial rate
( mol L−1
s-1
)
1 0.10 0.10 0.10 3.0×10−3
2 0.20 0.10 0.10 2.4×10−2
3 0.10 0.20 0.10 3.0×10−3
4 0.10 0.10 0.30 2.7×10−2
(a) rate = k[C]3
[D]0
[E]2
= k[C]3
[E]2
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-26-2048.jpg)
![27
Exp.
[C]
( mol L−1
)
[D]
( mol L−1
)
[E]
( mol L−1
)
Initial rate
( mol L−1
s-1
)
1 0.10 0.10 0.10 3.0×10−3
2 0.20 0.10 0.10 2.4×10−2
3 0.10 0.20 0.10 3.0×10−3
4 0.10 0.10 0.30 2.7×10−2
(b) rate = k[C]3
[E]2
From experiment 1,
3.0×10−3
= k(0.10)3
(0.10)2
k = 300 mol−4
L4
s−1
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-27-2048.jpg)
![28
Question
H+
CH3COCH3(aq) + I2(aq) CH3COCH2I(aq) + H+
(aq) + I−
(aq)
Initial rate
(mol L−1
s−1
)
Initial concentration
(mol L−1
)
[I2
(aq)] [CH3
COCH3
(aq)] [H+
(aq)]
3.5 ×10−5
2.5×10−4
2.0×10−1
5.0×10−3
3.5 ×10−5
1.5×10−4
2.0×10−1
5.0×10−3
1.4 ×10−4
2.5×10−4
4.0×10−1
1.0×10−2
7.0 ×10−5
2.5×10−4
4.0×10−1
5.0×10−3
(a) Suppose,
rate = k[I2(aq)]n
[CH3COCH3(aq)]m
[H+
(aq)]p
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-28-2048.jpg)
![29
Initial rate
(mol L−1
s−1
)
Initial concentration
(mol L−1
)
[I2
(aq)] [CH3
COCH3
(aq)] [H+
(aq)]
3.5 ×10−5
2.5×10−4
2.0×10−1
5.0×10−3
3.5 ×10−5
1.5×10−4
2.0×10−1
5.0×10−3
1.4 ×10−4
2.5×10−4
4.0×10−1
1.0×10−2
7.0 ×10−5
2.5×10−4
4.0×10−1
5.0×10−3
(a) rate = k[I2(aq)]n
[CH3COCH3(aq)]m
[H+
(aq)]p
p3-m1-n4-
p-3m-1n-4
5
-5
)10(5.0)10(2.0)10(1.5
)10(5.0)10(2.0)10(2.5
103.5
103.5
×××
×××
=
×
×
−
From experiments 1 and 2,
1 = 1.67n ⇒ n = 0
= 1.67n
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-29-2048.jpg)
![30
Initial rate
(mol L−1
s−1
)
Initial concentration
(mol L−1
)
[I2
(aq)] [CH3
COCH3
(aq)] [H+
(aq)]
3.5 ×10−5
2.5×10−4
2.0×10−1
5.0×10−3
3.5 ×10−5
1.5×10−4
2.0×10−1
5.0×10−3
1.4 ×10−4
2.5×10−4
4.0×10−1
1.0×10−2
7.0 ×10−5
2.5×10−4
4.0×10−1
5.0×10−3
(a) rate = k[I2(aq)]n
[CH3COCH3(aq)]m
[H+
(aq)]p
p3-m1-n4-
p-3m-1n-4
5
-5
)10(5.0)10(2.0)10(2.5
)10(5.0)10(4.0)10(2.5
103.5
107.0
×××
×××
=
×
×
−
From experiments 1 and 4,
2 = 2m ⇒m= 1
= 2m
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-30-2048.jpg)
![31
Initial rate
(mol L−1
s−1
)
Initial concentration
(mol L−1
)
[I2
(aq)] [CH3
COCH3
(aq)] [H+
(aq)]
3.5 ×10−5
2.5×10−4
2.0×10−1
5.0×10−3
3.5 ×10−5
1.5×10−4
2.0×10−1
5.0×10−3
1.4 ×10−4
2.5×10−4
4.0×10−1
1.0×10−2
7.0 ×10−5
2.5×10−4
4.0×10−1
5.0×10−3
(a) rate = k[I2(aq)]n
[CH3COCH3(aq)]m
[H+
(aq)]p
p3-m1-n4-
p-2m-1n-4
5
-4
)10(5.0)10(4.0)10(2.5
)10(1.0)10(4.0)10(2.5
107.0
101.4
×××
×××
=
×
×
−
From experiments 3 and 4,
2 = 2p ⇒ p = 1
= 2p
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-31-2048.jpg)
![32
Initial rate
(mol L−1
s−1
)
Initial concentration
(mol L−1
)
[I2
(aq)] [CH3
COCH3
(aq)] [H+
(aq)]
3.5 ×10−5
2.5×10−4
2.0×10−1
5.0×10−3
3.5 ×10−5
1.5×10−4
2.0×10−1
5.0×10−3
1.4 ×10−4
2.5×10−4
4.0×10−1
1.0×10−2
7.0 ×10−5
2.5×10−4
4.0×10−1
5.0×10−3
(a) Rate = k[I2(aq)]0
[CH3COCH3(aq)][H+
(aq)]
= k[CH3COCH3(aq)][H+
(aq)]
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-32-2048.jpg)
![33
Initial rate
(mol L−1
s−1
)
Initial concentration
(mol L−1
)
[I2
(aq)] [CH3
COCH3
(aq)] [H+
(aq)]
3.5 ×10−5
2.5×10−4
2.0×10−1
5.0×10−3
3.5 ×10−5
1.5×10−4
2.0×10−1
5.0×10−3
1.4 ×10−4
2.5×10−4
4.0×10−1
1.0×10−2
7.0 ×10−5
2.5×10−4
4.0×10−1
5.0×10−3
(b) Rate = k[CH3COCH3(aq)][H+
(aq)]
From experiment 1,
3.5×10−5
= k(2.0×10−1
)(5.0×10−3
)
k = 0.035 mol−1
L1
s−1
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-33-2048.jpg)

![36
A → products
n
k[A]
dt
d[A]
Rate =−=
(Differential rate equation)
shows the variation of rate with [A]
Two types of plots to determine k and n
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-36-2048.jpg)
![37
[A]
rate
n
k[A]
dt
d[A]
rate =−=
n = 0k
rate = k
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
Concentration of reactant A](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-37-2048.jpg)
![38
Examples of zero-order reactions : -
2NH3(g) N2(g) + 3H2(g)
Fe or W as catalyst
Decomposition of NH3/HI can take place only
on the surface of the catalyst.
Once the surface is covered completely
(saturated) with NH3/HI molecules at a given
concentration of NH3/HI, further increase in
[NH3]/[HI] has no effect on the rate of
reaction.
2HI(g) H2(g) + I2(g)
Au as catalyst
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-38-2048.jpg)
![39
[A]
rate
nk
dt
d[A]
rate [A]=−=
n = 1
slope = k
linear
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
Concentration of reactant A](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-39-2048.jpg)
![40
[A]
rate
2
k[A]
dt
d[A]
rate =−=
n = 2
k cannot be determined directly
from the graph
parabola
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
Concentration of reactant A](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-40-2048.jpg)
![41
n
k[A]
dt
d[A]
rate =−=
[A]
rate n = 2
n = 1
n = 0
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
Concentration of reactant A](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-41-2048.jpg)
![42 log10[A]
log10rate
n
k[A]rate =
n
1010 k[A]logratelog =
slope
y-intercept
n = 0
n = 1
log10k
n = 2
[A]nlogklog 1010 +=
slope = 1
slope = 2
slope = 0
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-42-2048.jpg)
![43
n
k[A]
dt
d[A]
=− (Differential rate equation)
kdtd[A] −=
∫ ∫−=
t
0 0
A
A
t
t
dtkd[A]
kt[A][A] 0t −=−
[A]t = [A]0 – kt (Integrated rate
equation)
If n = 0
Derivation not required
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-43-2048.jpg)
![44
[A]t = [A]0 – kt (Integrated rate equation)
shows variation of [A] with time
time
[A]t
ratek
dt
d[A]
slope −=−==
[A]0
constant rate
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
ConcentrationofreactantA
t
AA
k t][][ 0 −
=](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-44-2048.jpg)
![45
n
k[A]
dt
d[A]
=− (Differential rate equation)
(Integrated rate
equation)
If n = 1, k[A]
dt
d[A]
=−
kdt
[A]
d[A]
−=
∫ ∫−=
t
0 0
[A]
[A]
t
t
dtkd[A]
[A]
1
loge[A]t – loge[A]0 = −kt
Or [A]t = [A]0 e−kt
loge[A]t = loge[A]0 − kt
ln
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-45-2048.jpg)
![46
Two types of plots to determine k and n
Or [A]t = [A]0 e−kt
loge[A]t = loge[A]0 − kt
time
log[A]t
log[A]0
slope = −k/2.303 k= -2.303xslpoe
linear → n = 1
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
][
][
log
303.2 0
tA
A
t
k =
intercept = log[A]0
[A]0=antilog (intercept)](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-46-2048.jpg)
![47
Two types of plots to determine k and n
Or [A]t = [A]0 e−kt
loge[A]t = loge[A]0 − kt
time
[A]t
[A]t varies exponentially with time
constant half life → n = 1
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-47-2048.jpg)
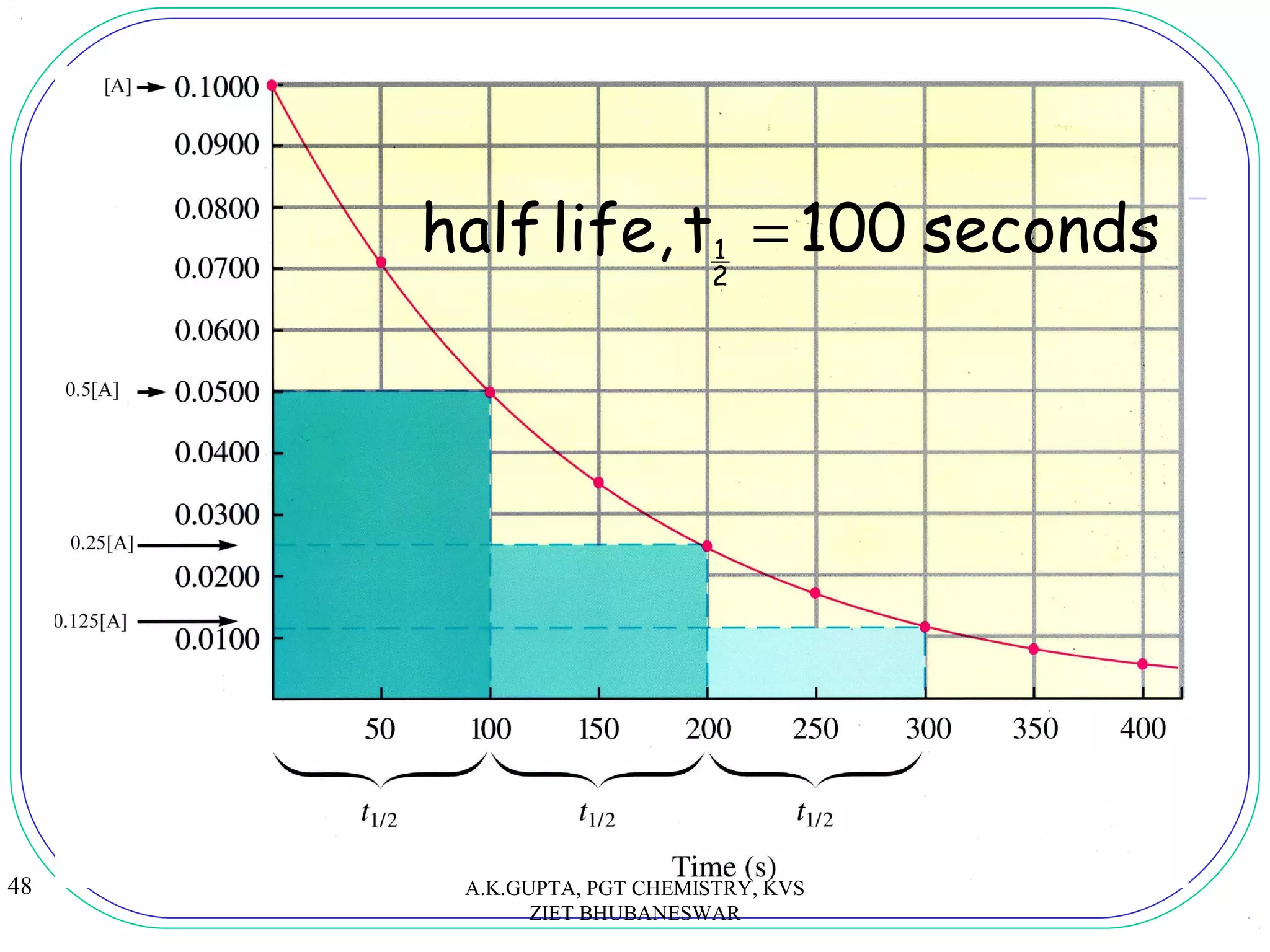
![49
2
1ttwhen = 0t [A]
2
1
[A] =
693.0301.0303.2
k
log2
303.2t
2
1 === x
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
tA
A
t
k
][
][
log
303.2 0
=](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-49-2048.jpg)

![51
Question
For hydrolysis
sucrose → fructose + glucose
Rate = k[sucrose] k = 0.208 h−1
at 298 K
a. Determine the rate constant of the
reaction.
b. Calculate the time in which 87.5%of
sucrose has decomposed
(a) h3.33
h0.208
12.303x0.30
k
2.303log2
t 12
1 === −
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-51-2048.jpg)
![52
Q.17
sucrose → fructose + glucose
Rate = k[sucrose] k = 0.208 h−1
at 298 K
(b)
87.5% decomposed → [A]t = 0.125[A]0
On solving we get = 9.99 h
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR
tA
A
t
k
][
][
log
303.2 0
=
0
0
][125.0
][
log
303.2
A
A
k
t =](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-52-2048.jpg)
![53
mol−1
L1
s−1
kagainst t2
s−1−k/2.303log[A]t
against t1
mol L−1
s−1−k[A]t
against t[A]t
= [A]0
– kt0
Units of kSlope
Straight line
plot
Integrated
rate
equation
Order
t[A]
1
kt
[A]
1
[A]
1
0t
+=
kt/2.303
[A]
[A]
log
0
t
−=
Summary : - For reactions of the type
A → Products
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-53-2048.jpg)
![54
2H2O2(aq) → 2H2O(l) + O2(g)
Rate = k[H2O2(aq)]
Examples of First Order ReactionsExamples of First Order Reactions
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-54-2048.jpg)
![55
Examples of First Order ReactionsExamples of First Order Reactions
Reaction Rate equation
2N2O5(g) → 4NO2(g) + O2(g) Rate = k[N2O5(g)]
SO2Cl2(l) → SO2(g) + Cl2(g) Rate = k[SO2Cl2(l)]
(CH3)3CCl(l) + OH-
(aq)
→ (CH3)3COH(l) + Cl-
(aq)
Rate = k[(CH3)3CCl(l)]
(SN1)
All radioactive decays e.g. Rate = k[Ra]
SN1 : 1st
order Nucleophilic Substitution Reaction
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-55-2048.jpg)
![56
1. For a reaction involving one reactant only:
2NOCl(g) → 2NO(g) + Cl2(g)
Rate = k[NOCl(g)]2
2NO2(g) → 2NO(g) + O2(g)
Rate = k[NO2(g)]2
Examples of Second Order ReactionsExamples of Second Order Reactions
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-56-2048.jpg)
![57
Examples of Second Order ReactionsExamples of Second Order Reactions
Reaction Rate equation
H2(g) + I2(g) → 2HI(g) Rate = k[H2(g)][I2(g)]
CH3Br(l) + OH−
(aq)
→ CH3OH(l) + Br−
(aq)
Rate = k[CH3Br(l)][OH−
(aq)] (SN2)
CH3COOC2H5(l) + OH−
(aq)
→ CH3COO−
(aq) + C2H5OH(l)
Rate = k[CH3COOC2H5(l)][OH−
(aq)]
SN2 : 2nd
order Nucleophilic Substitution Reaction
2. For a reaction involving one reactant only:
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-57-2048.jpg)
![58
2. For a reaction involving two reactants:
A + B → products
Rate = k[A][B]
To determine the rate equation, the concentration
of one of the reactants must be kept constant
(in large excess) such that the order of reaction
w.r.t. the other reactant can be determined.
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-58-2048.jpg)
![59
2. For a reaction involving two reactants:
A + B → products
Rate = k[A][B]
When [B] is kept constant,
excess
rate = k’[A] (where k’ = k[B]excess)
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-59-2048.jpg)
![60
Rate = k[A][B]excess = k’[A]
k can be determined from k’ if [B]excess is known
Linear → first order
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-60-2048.jpg)
![61
2. For a reaction involving two reactants:
A + B → products
Rate = k[B][A]
• When [A] is kept constant,
rate = k”[B] (where k” = k[A]excess)
excess
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-61-2048.jpg)
![62
Rate = k[A]excess[B] = k’’[B]
k can be determined from k’’ if [A]excess is known
Linear → first order
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-62-2048.jpg)
![63
Rate Equations and Order of Reactions
(a) The reaction between tyrosine (an amino acid) and
iodine obeys the rate law: rate = k [Tyr] [I2].
Write the orders of the reaction with respect to tyrosine
and iodine respectively, and hence the overall order.
Answer(a) The order of the reaction with respect to
tyrosine is 1, and the order of the reaction
with respect to iodine is also 1. Therefore, the
overall order of the reaction is 2.
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-63-2048.jpg)
![64
Rate Equations and Order of Reactions
(b) Determine the unit of the rate constant (k) of the
following rate equation:
Rate = k [A] [B]3
[C]2
(Assume that all concentrations are measured in
mol dm–3
and time is measured in minutes.)
Answer
(b) k =
∴ Unit of k =
= mol-5
dm15
min-1
23
]C[]B][A[
Rate
63-
-1-3
)dm(mol
mindmmol
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-64-2048.jpg)



![68
Determination of Simple Rate Equations from Initial Rate Method
(a) Let x be the order of reaction with respect to A, and y be the
order of reaction with respect to B. Then, the rate equation for
the reaction can be expressed as:
Rate = k [A]x
[B]y
Therefore,
0.0005 = k (0.01)x
(0.02)y
.......................... (1)
0.0010 = k (0.02)x
(0.02)y
.......................... (2)
0.002 0 = k (0.01)x
(0.04)y
.......................... (3)
Dividing (1) by (2),
∴ x = 1
x
)
02.0
01.0
(
0010.0
50.000
=
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-68-2048.jpg)

![70
Determination of Simple Rate Equations from Initial Rate Method
(b) Using the result of experiment (1),
Rate = k [A] [B]2
0.000 5 = k × 0.01 × 0.022
k = 125 mol-2
dm6
s-1
(c) Rate = 125 [A] [B]2
(b) Calculate the rate constant using the result of
experiment 1.
(c) Write the rate equation for the reaction.
Answer
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-70-2048.jpg)


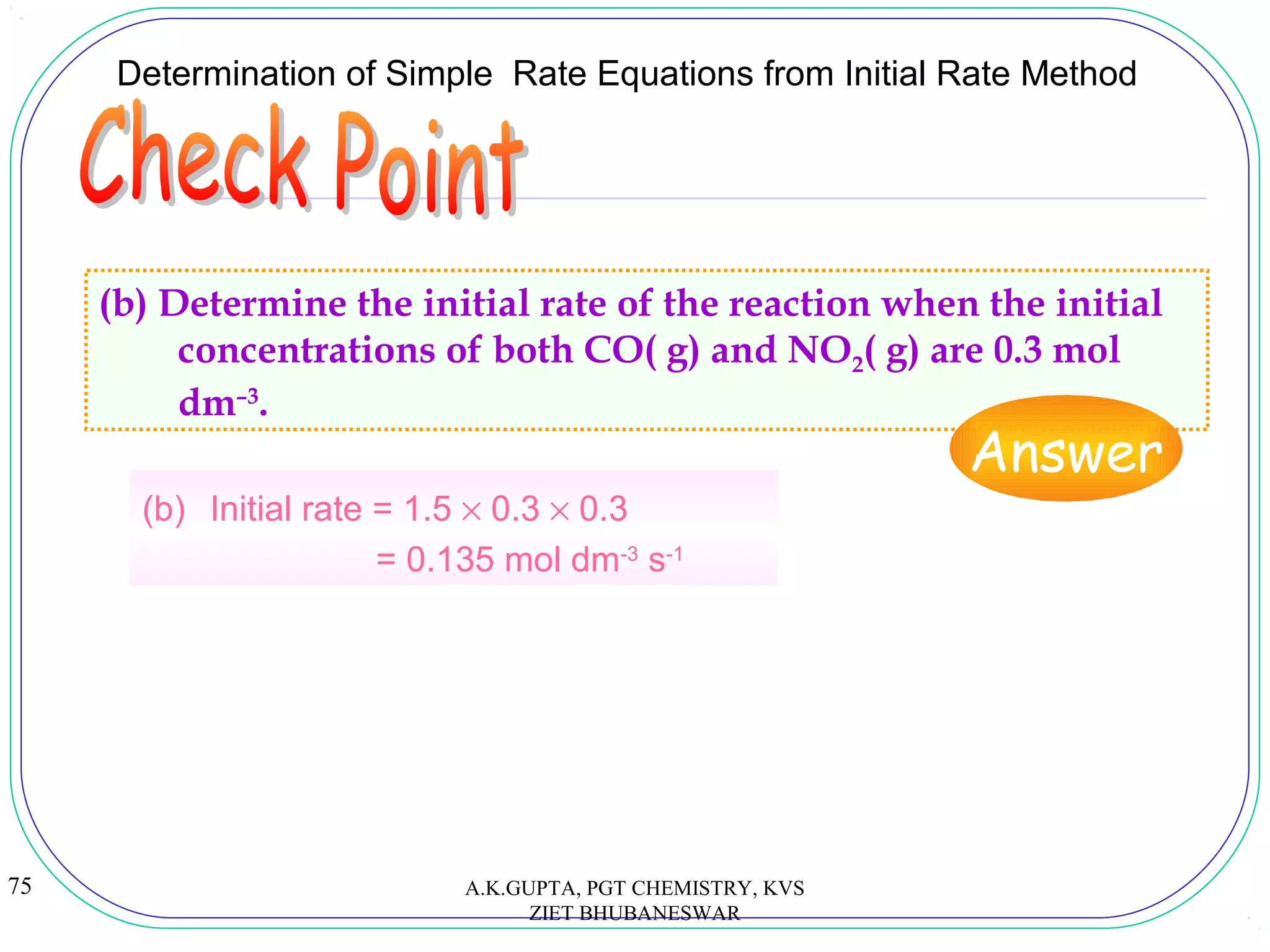
![73
14.3 Determination of Simple Rate Equations from Initial Rate Method
(SB p.31)
(a) Let m be the order of reaction with respect to CO, and n be the
order of reaction with respect to NO2. Then, the rate equation for
the reaction can be expressed as:
Rate = k [CO]m
[NO2]n
Therefore,
0.015 = k (0.1)m
(0.1)n
.......................... (1)
0.030 = k (0.2)m
(0.1)n
.......................... (2)
0.030 = k (0.1)m
(0.2)n
.......................... (3)
Dividing (1) by (2),
∴ m = 1
m
)
2.0
1.0
(
030.0
0.015
=
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-73-2048.jpg)
![74
Determination of Simple Rate Equations from Initial Rate Method
(a) Dividing (1) by (3),
∴ n = 1
∴ Rate = k [CO] [NO2]
Using the result of experiment (1),
0.015 = k (0.1)2
k = 1.5 mol-1
dm3
s-1
∴ Rate = 1.5 [CO] [NO2]
n
)
2.0
1.0
(
030.0
0.015
=
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-74-2048.jpg)



![79
(c) The table below shows the initial rates of
decomposition of hydrogen peroxide solution of
different concentrations. Plot a graph of the initial
rate against [H2O2(aq)].
Determination of Simple Rate Equations from Differential Rate Equations
Answer
[H2O2(aq)]
(mol L-1
)
0.100 0.175 0.250 0.300
Initial rate
(10-4
mol L-1
s-1
)
0.59 1.04 1.50 1.80
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-79-2048.jpg)


![82
Determination of Simple Rate Equations from Differential Rate Equations
(d) There are two methods to determine the order and rate constant
of the reaction.
Method 1:
When the concentration of hydrogen peroxide solution
increases from 0.1 mol dm–3
to 0.2 mol dm–3
, the reaction rate
increases from 0.59 × 10–4
mol dm–3
s–1
to about 1.20 × 10–4
mol dm–3
s–1
.
∴ Rate ∝ [H2O2(aq)]
Therefore, the reaction is of first order.
The rate constant (k) is equal to the slope of the graph.
k =
= 6.0 × 10-4
s-1
3-
-1-3
dmmol0)-.3000(
sdmmol0)-4-10(1.8×
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-82-2048.jpg)
![83
Determination of Simple Rate Equations from Differential Rate Equations
(d) Method 2:
The rate equation can be expressed as:
Rate = k [H2O2(aq)]x
where k is the rate constant and x is the order of reaction.
Taking logarithms on both sides of the rate equation,
log (rate) = log k + x log [H2O2(aq)] ................. (1)
-3.74-3.82-3.98-4.23log (rate)
-0.523-0.602-0.757-1.000log
[H2O2(aq)]
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-83-2048.jpg)
![84
Determination of Simple Rate Equations from Differential Rate Equations
(d) A graph of log (rate) against log [H2O2(aq)] gives a straight line
of slope x and y-intercept log k.
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-84-2048.jpg)
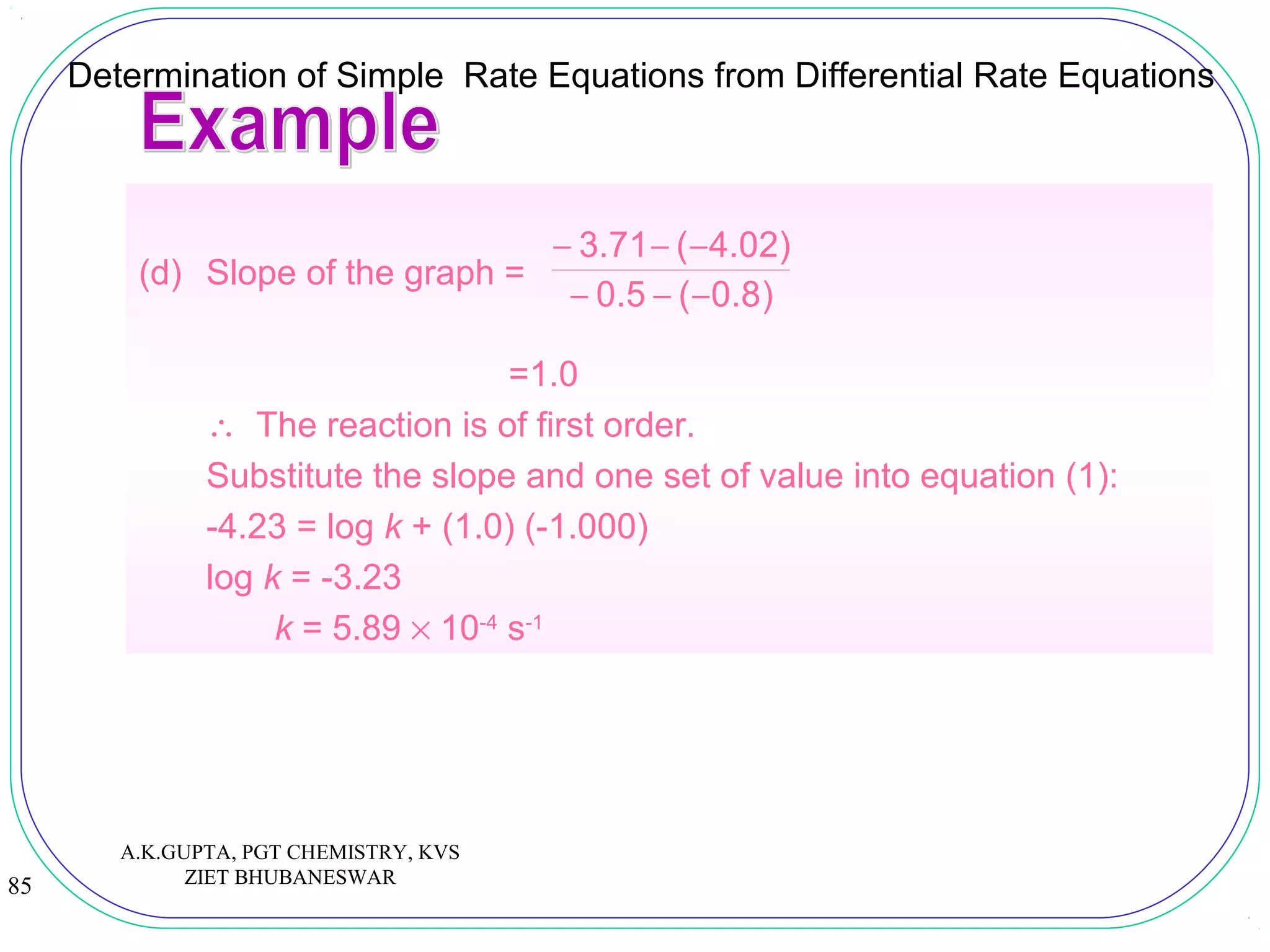


![88
Determination of Simple Rate Equations from Differential Rate Equations
(i) Write the rate equation for the reaction.
Answer
(i) The rate equation for the reaction can be
expressed as:
Rate = k [N2O5(g)]m
where k is the rate constant and m is the
order of reaction.
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-88-2048.jpg)
![90
Determination of Simple Rate Equations from Differential Rate Equations
(ii) Method 1:
A graph of the initial rates against [N2O5(g)] is shown as follows:
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-90-2048.jpg)
![91
Determination of Simple Rate Equations from Differential Rate Equations
As shown in the graph, when the concentration of N2O5 increases
from 1.0 × 10–3
mol dm–3
to 2.0 × 10–3
mol dm–3
, the rate of the
reaction increases from 0.075 mol dm–3
s–1
to 0.15 mol dm–3
s–1
.
∴ Rate ∝ [N2O5(g)]
∴ The reaction is of first order.
Then, the rate constant k is equal to the slope of the graph.
k =
= 75 s-1
∴ The rate equation for the reaction is:
Rate = 75 [N2O5(g)]
1-3-3-
-1-3
sdmmol0)-10.61(
sdmmol0)(0.12
×
−
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-91-2048.jpg)

![93
Determination of Simple Rate Equations from Differential Rate Equations
(iii) The rate equation, rate = 75 [N2O5(g)], is used for the
following calculation.
(1) Rate = 75 [N2O5(g)]
= 75 s–1
× 2.0 × 10–3
mol dm–3
= 0.15 mol dm–3
s–1
(2) Rate = 75 [N2O5(g)]
= 75 s–1
× 2.4 × 10–2
mol dm–3
= 1.8 mol dm–3
s–1
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-93-2048.jpg)

![95
Determination of Simple Rate Equations from Integrated Rate Equations
As radioactive decay is a first order reaction,
= 3.47 × 10-4
year-1
∴ t = 4638 years
∴ It takes 4638 years for the radioactivity of a sample of A to
dropt to 20 % of its original level.
k
t
693.0
2
1 =
1997
693.0
=k
kt=)
[A]
[A]
(ln 0
t4
10473.)
%20
%100
(ln −
×=
A.K.GUPTA, PGT CHEMISTRY, KVS
ZIET BHUBANESWAR](https://image.slidesharecdn.com/chemicalkinetics-akg-160627100659/75/Chemical-kinetics-95-2048.jpg)



