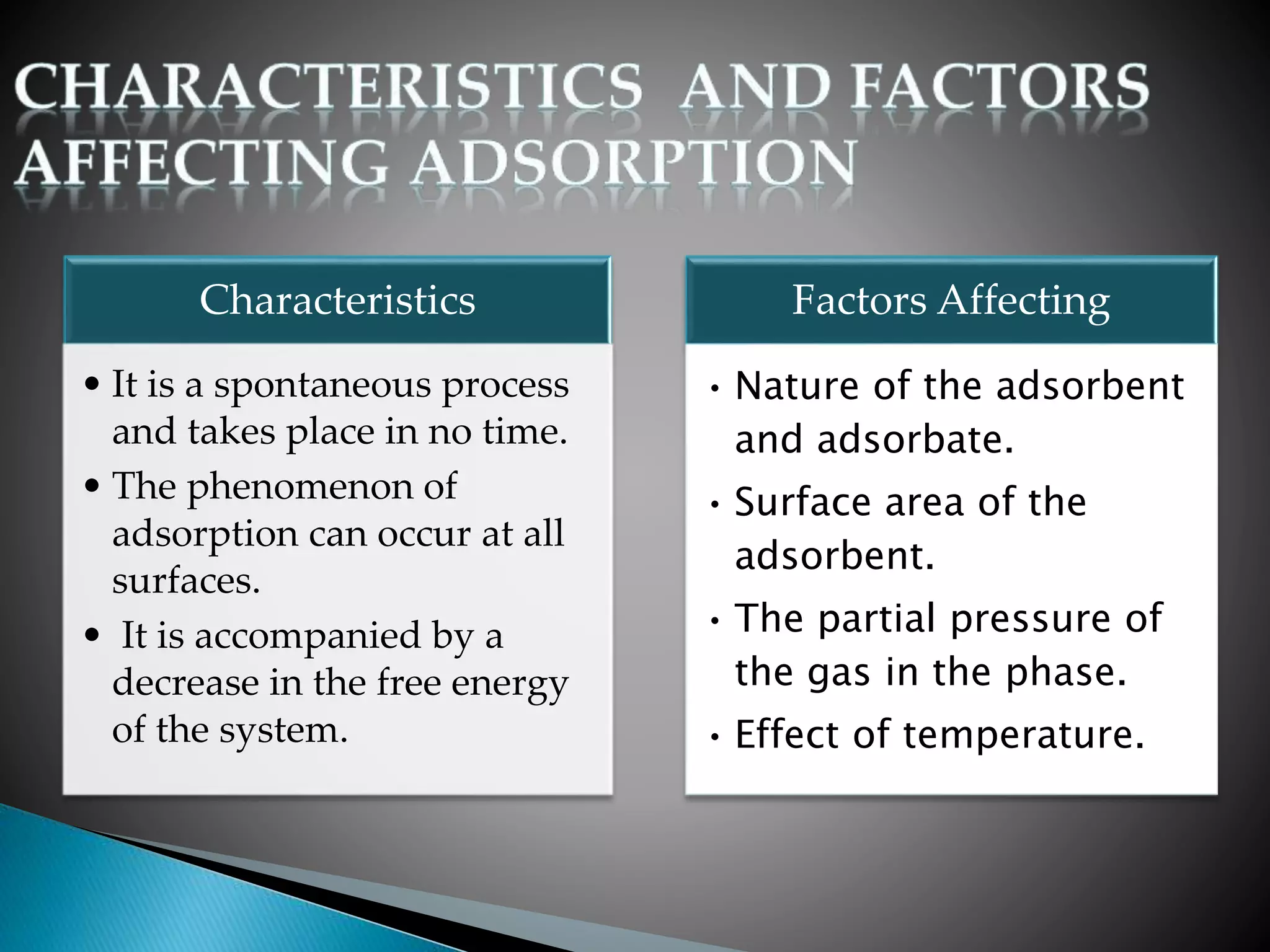
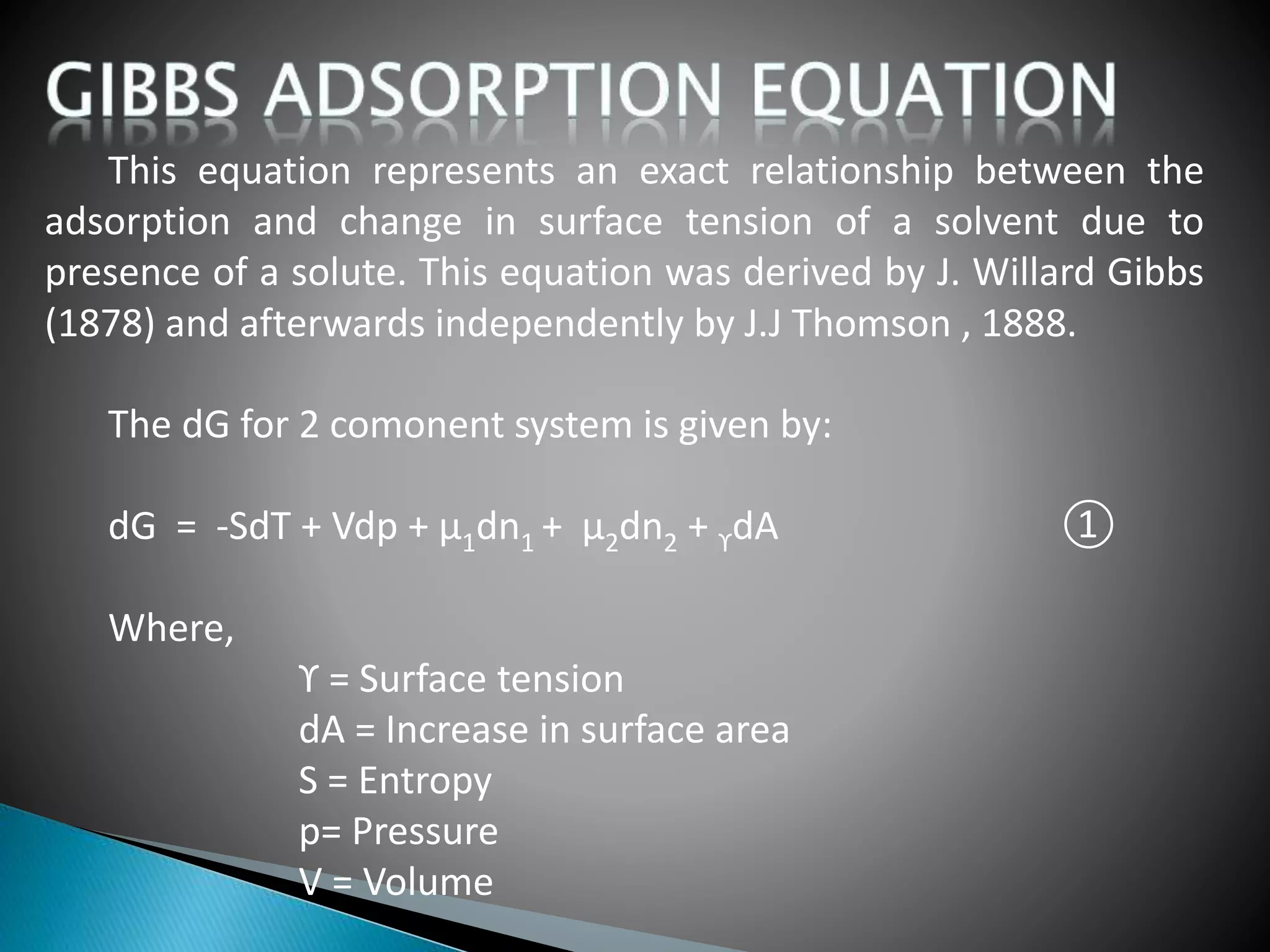
The document discusses adsorption and the Gibbs adsorption equation. It defines adsorption as molecules of a gas or liquid being attracted to and retained on the surface of a solid. Absorption occurs when a substance is uniformly distributed throughout a solid or liquid. The Gibbs adsorption equation relates changes in surface tension to changes in solute concentration at an interface. It shows that the surface excess concentration of a solute is proportional to the negative of the derivative of surface tension with respect to solute activity. The document also lists factors that affect adsorption and describes adsorption isotherms.





![Where
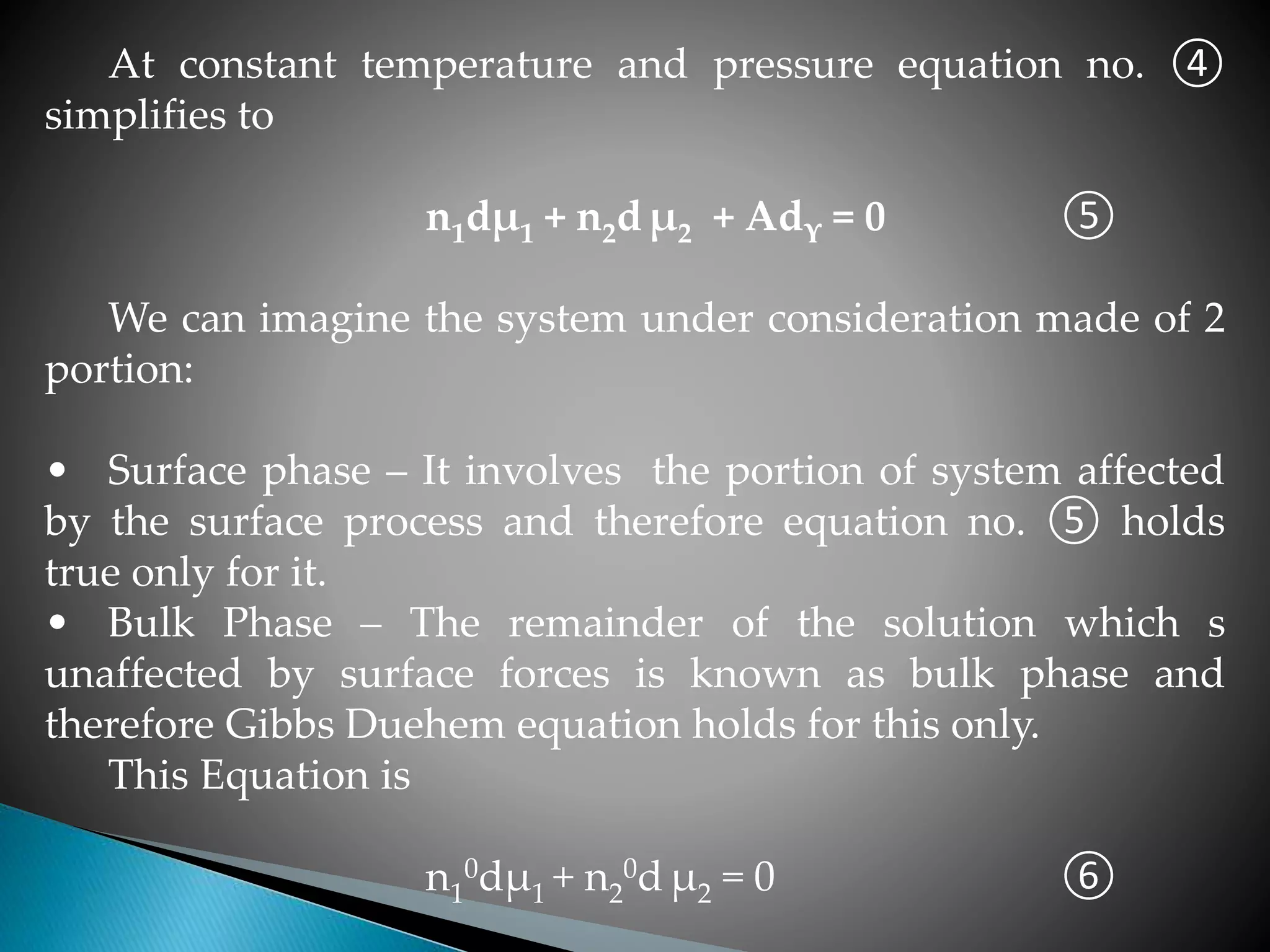
On multiplying equation ⑥ by n1/n1
0 and subtracting from
equation no. ⑤ we obtain the expression
Adϒ + (n2 – n1n2
0/n1
0) dµ2 = 0
“Or”
-dϒ/dµ = [n2 - n1n2
0/n1
0]/A ⑦
Where,
n2 Represents no. of moles of solute associated with n1 moles of
solvent in the surface phase and n1n2
0/ n1
0 is the corresponding
quantity in the bulk phase. It therefore, follows that the quantity [n2
- n1n2
0/n1
0]/A is the excess concentration of the solute per unit area
of the surface and is usually designated by the symbol ‘ᴦ’
Thus equation no. 7 becomes as
ᴦ = -dϒ/ dµ2 ⑧](https://image.slidesharecdn.com/gibbsadsorptionisotherm-130714004658-phpapp02-151214085912/75/Gibbs-Adsorption-Isotherm-9-2048.jpg)
![Where ᴦ is independent of n1 and is dependent only on the nature of surface phase
and not on its amount.
ᴦ is also called the surface concentration of solute per unit area of interface .
For a solution :
µ2 = µ2
0 + RT ln a2 ⑨
Where,
a2 is the activity of solute
By differentiating equation no. ⑨ we get:
dµ= RT d ln a2 ⑩
Assuming µ2
0 as a constant
On substituting eq. no. ⑩ in eq. no. ⑧ we get,
ᴦ= 1/ RT ₓ dϒ/d ln a2
or
ᴦ= -a2/ RT ₓ dϒ/da2 [Since d ln a2 = da2/a2 ] ⑪
Equation no. ⑪ is known as Gibbs Adsorption Equation.](https://image.slidesharecdn.com/gibbsadsorptionisotherm-130714004658-phpapp02-151214085912/75/Gibbs-Adsorption-Isotherm-10-2048.jpg)

