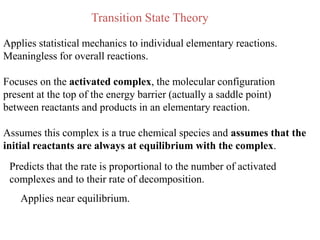
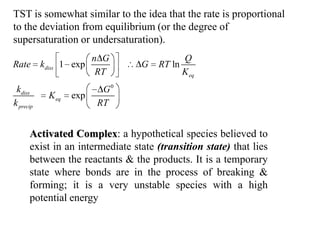
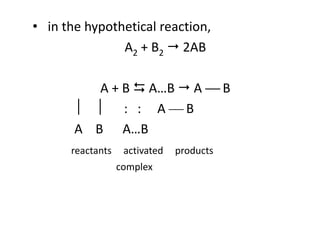
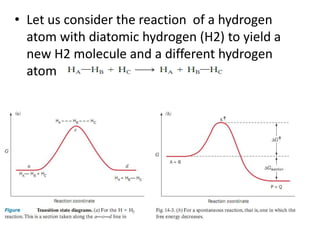
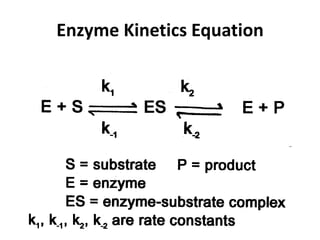
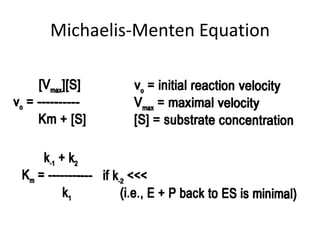
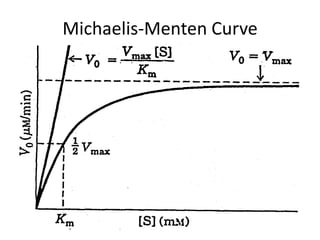
This document discusses enzyme kinetics and the Michaelis-Menten model of enzyme kinetics. It defines key terms like reaction rate, elementary reactions, rate laws, and transition state theory. It then introduces the Michaelis-Menten equation, defines terms like Km, Vmax, and kcat. It discusses steady state kinetics and how the Michaelis-Menten equation was derived. It explains the meaning and uses of Km and Vmax and concludes by discussing the Lineweaver-Burk double reciprocal plot.

![Reaction Rate Defined
Reaction rate: changes in a
concentration of a product or a
[]
reactant per unit time.
[ ] concentration
Reaction rate = ——
t change [
]
t
t](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-5-320.jpg)
![Expressing reaction rates
For a chemical reaction, there are many ways to express the
reaction rate. The relationships among expressions depend on
the equation.
Note the expression and reasons for their relations for the
reaction
2 NO + O2 (g) = 2 NO2 (g)
[O2] 1 [NO] 1 [NO2]
Reaction rate = – ——— = – — ———— = — ———
t 2 t 2 t](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-6-320.jpg)

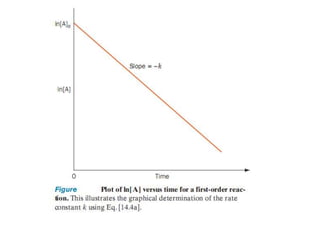
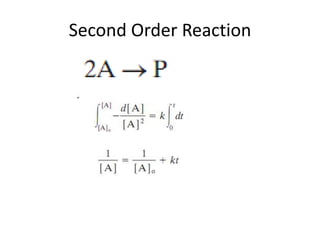
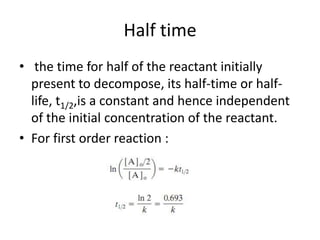
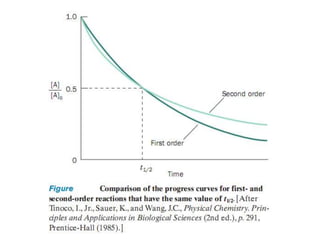
![Variation of Reaction rates and Order
2nd order, rate = k [A]2
rate
First order, rate = k [A]
k = rate, 0th
order
[A]
[A] = ___?](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-12-320.jpg)
![Initial Velocity (vo) and [S]
• The concentration of substrate [S] present will greatly
influence the rate of product formation, termed the
velocity (v) of a reaction. Studying the effects of [S] on
the velocity of a reaction is complicated by the
reversibility of enzyme reactions, e.g. conversion of
product back to substrate. To overcome this problem,
the use of initial velocity (vo) measurements are used.
At the start of a reaction, [S] is in large excess of [P],
thus the initial velocity of the reaction will be
dependent on substrate concentration](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-19-320.jpg)
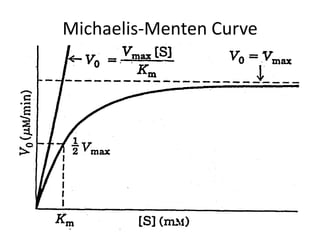
![Initial Velocity (vo) and [S] (cont)
• When initial velocity is plotted against [S], a
hyperbolic curve results, where Vmax
represents the maximum reaction velocity. At
this point in the reaction, if [S] >> E, all
available enzyme is "saturated" with bound
substrate, meaning only the ES complex is
present.](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-21-320.jpg)
![Substrate Saturation of an Enzyme
A. Low [S] B. 50% [S] or Km C. High, saturating [S]](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-23-320.jpg)
![Steady State Assumption
• The M-M equation was derived in part by making
several assumptions. An important one was: the
concentration of substrate must be much greater than
the enzyme concentration. In the situation where [S]
>> [E] and at initial velocity rates, it is assumed that the
changes in the concentration of the intermediate ES
complex are very small over time (vo). This condition is
termed a steady-state rate, and is referred to as
steady-state kinetics. Therefore, it follows that the
rate of ES formation will be equal to the rate ES
breakdown.](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-24-320.jpg)
![Michaelis-Menten Equation
Derivation
• Rate of ES formation = k1([ET] - [ES])[S]
(where [ET] is total concentration of
enzyme E and k-2 is considered neglible)
• Rate of ES breakdown to product = k-
1[ES] + k2[ES]](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-25-320.jpg)
![Michaelis-Menten Equation
Derivation (cont)
• Thus for the steady state assumption:
• k1([ET] - [ES])[S] = k-1[ES] + k2[ES]
• This equation is the basis for the final Michaelis-
Menten following algebraic rearrangement and
substitution of Km and Vmax terms.](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-26-320.jpg)
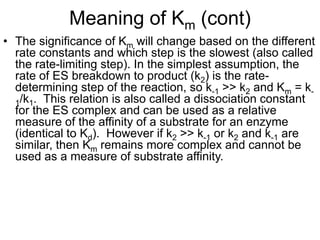
![Meaning of Km
• An important relationship that can be derived from the
Michaelis-Menten equation is the following: If vo is set
equal to 1/2 Vmax, then the relation Vmax /2 = Vmax[S]/Km
+ [S] can be simplied to Km + [S] = 2[S], or Km = [S].
This means that at one half of the maximal
velocity, the substrate concentration at this
velocity will be equal to the Km. This relationship
has been shown experimentally to be valid for many
enzymes much more complex in regards to the number of
substrates and catalytic steps than the simple single
substrate model used to derive it.](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-27-320.jpg)

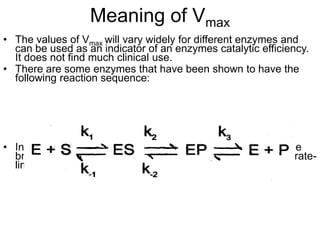
![Important Conclusions of Michaels -
Menten Kinetics
• when [S]= KM, the equation reduces to
• when [S] >> KM, the equation reduces to
• when [S] << KM, the equation reduces to](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-31-320.jpg)
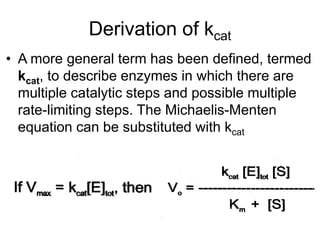
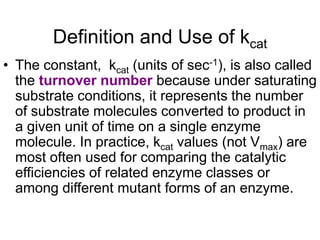
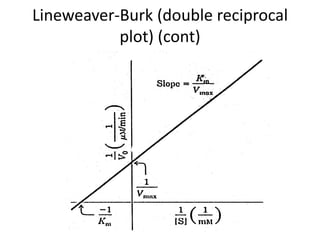
![Lineweaver-Burk (double reciprocal
plot)
• If the reciprocal (1/X) of the Michaelis-Menten
equation is done, after algebraic simplification the
following equation results:
• This relation is written in the format of the equation
for a straight line, y = mx + b, where y = 1/vo, m
(slope) = Km/Vmax, x = 1/[S] and the y-intercept, b
= 1/Vmax. When this relation is plotted,the result is
a straight line graph](https://image.slidesharecdn.com/enzymekinetics-120109001033-phpapp01/85/Enzyme-kinetics-34-320.jpg)