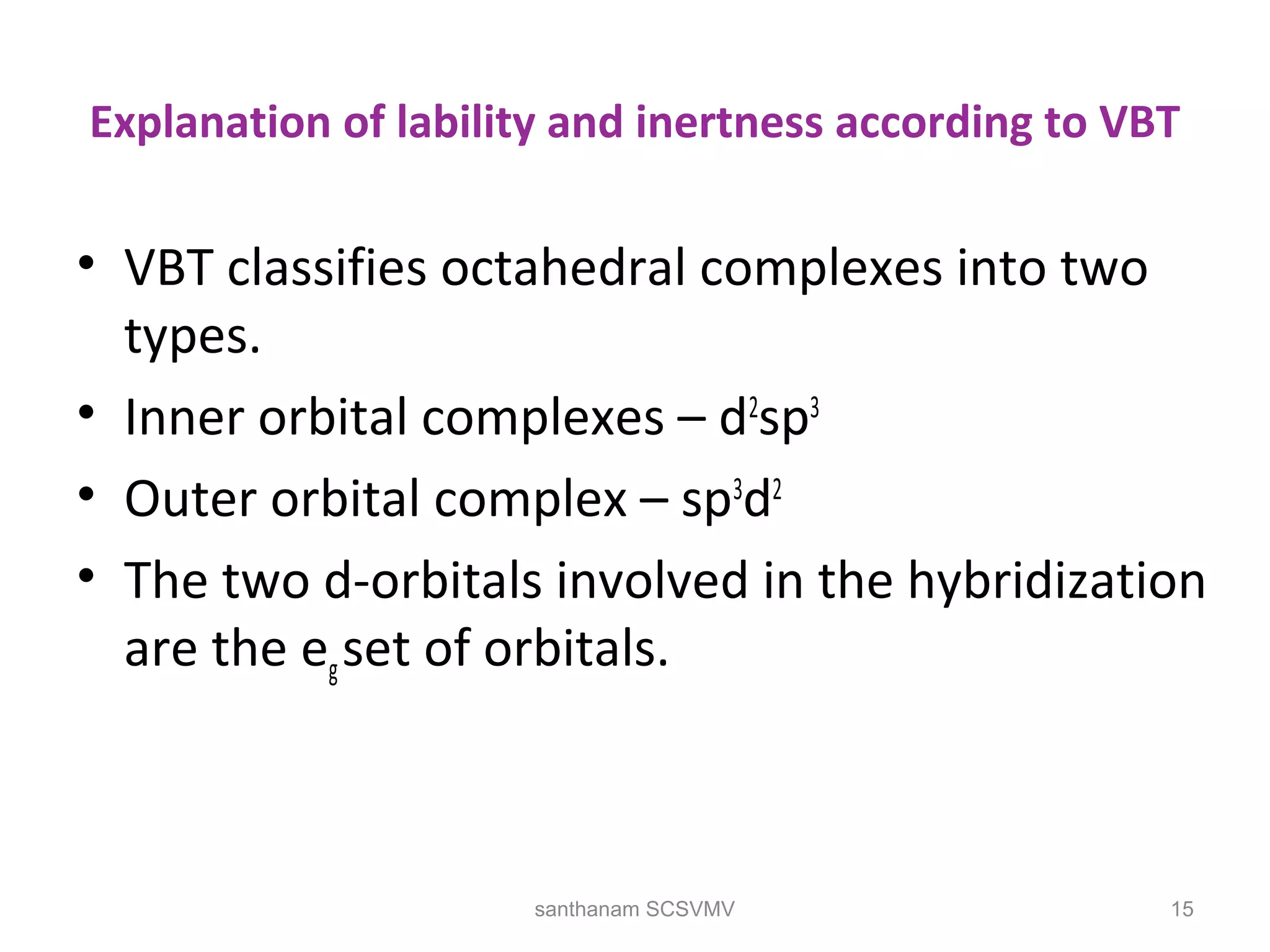
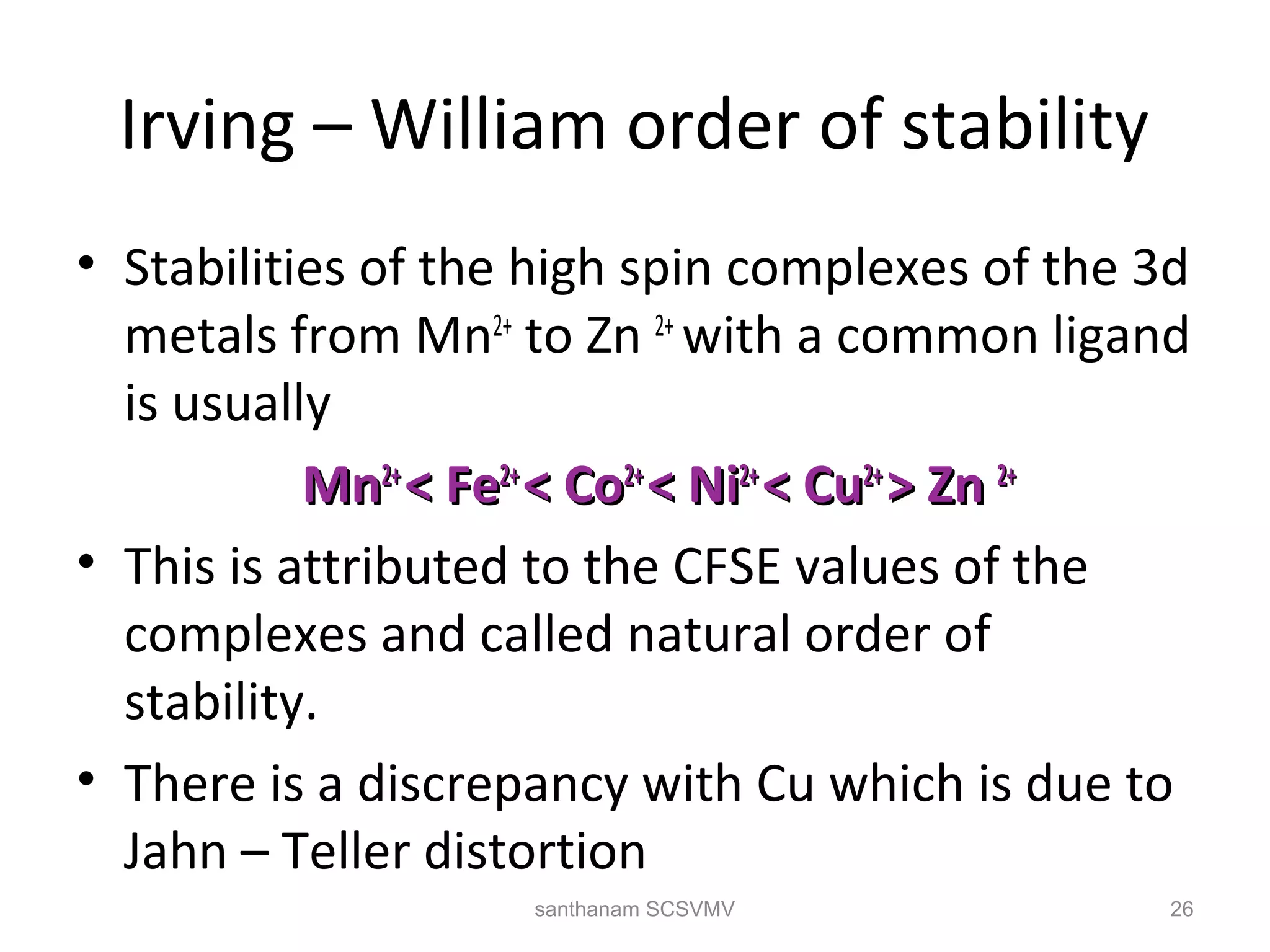
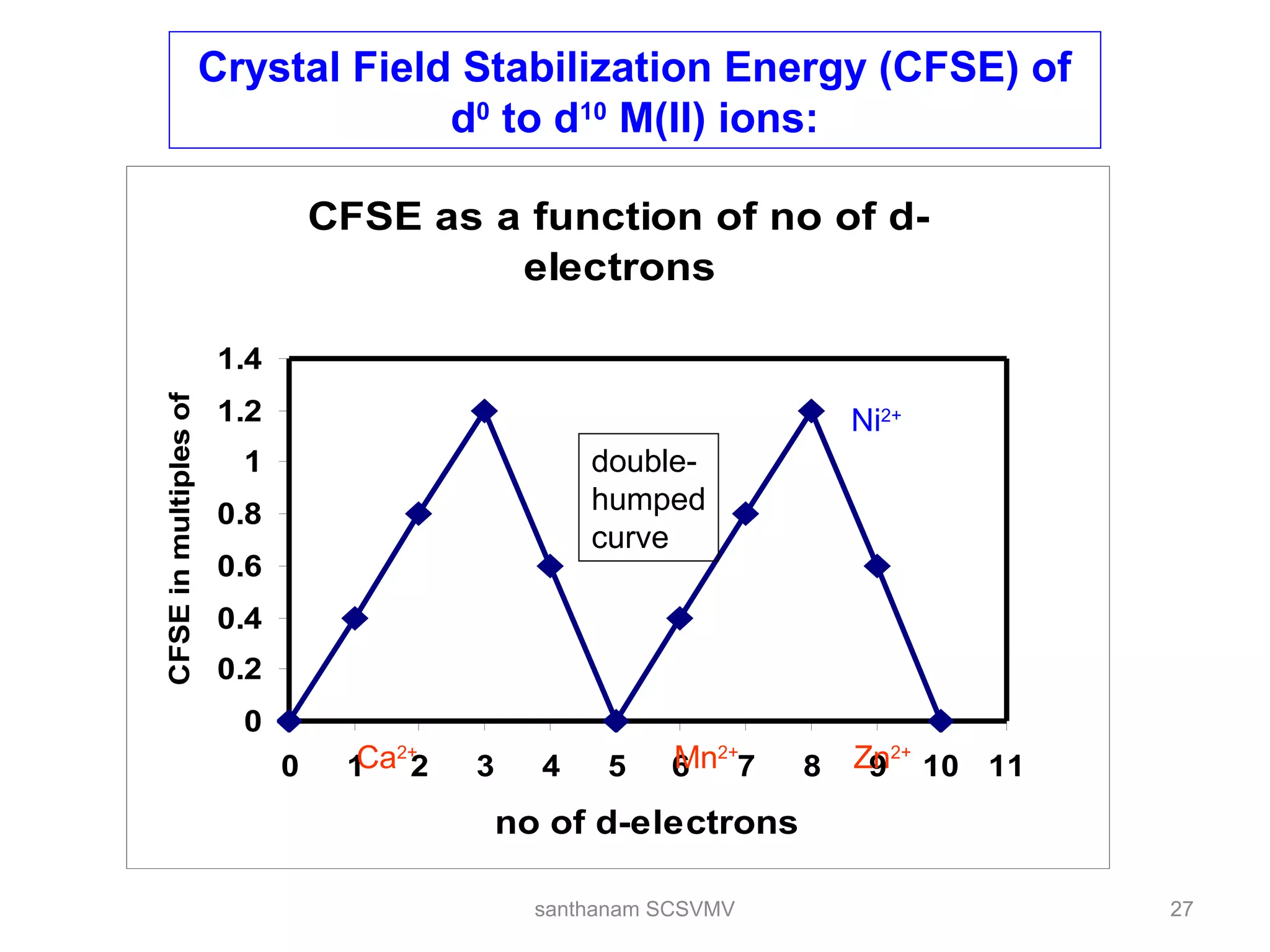
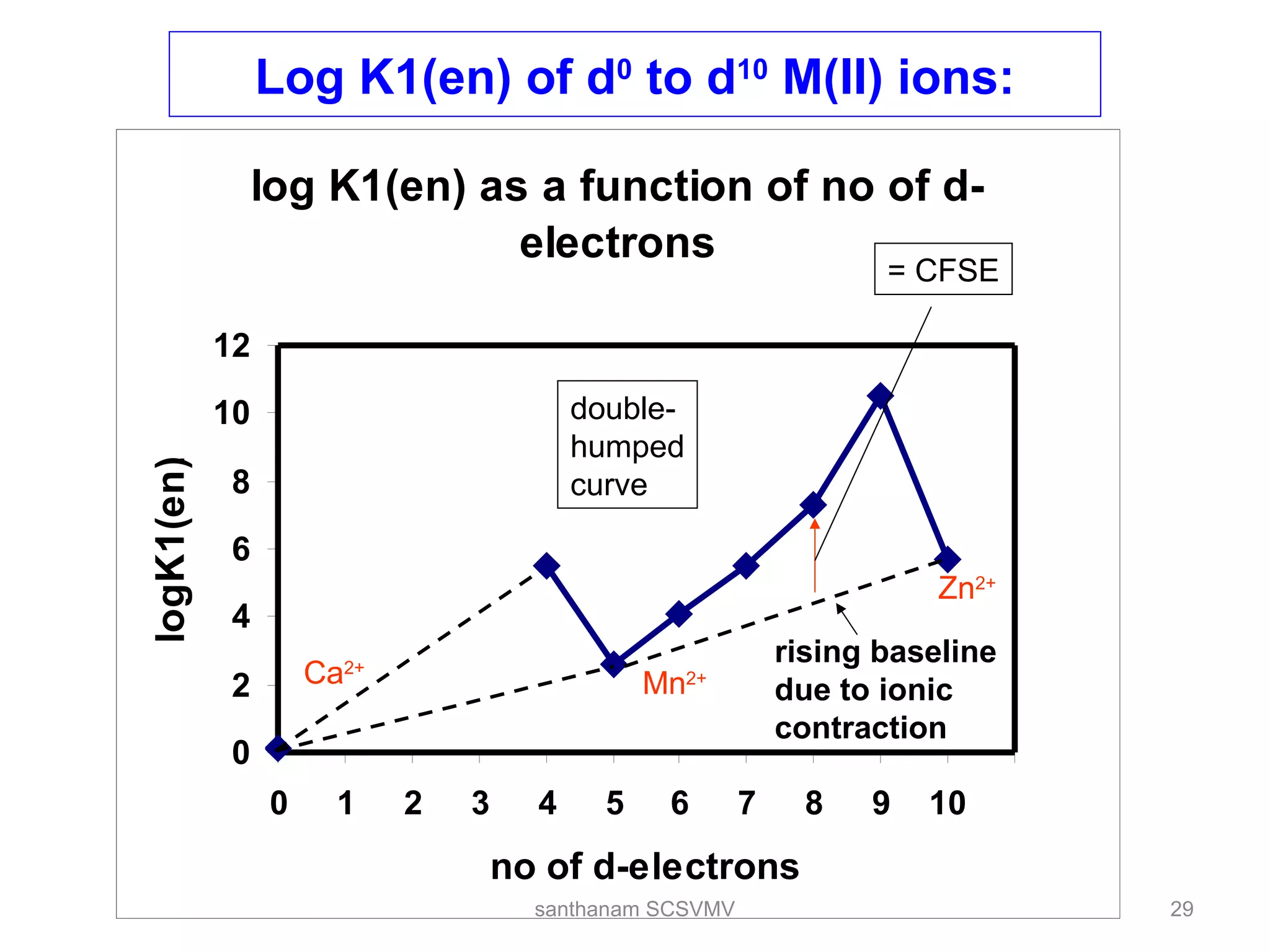
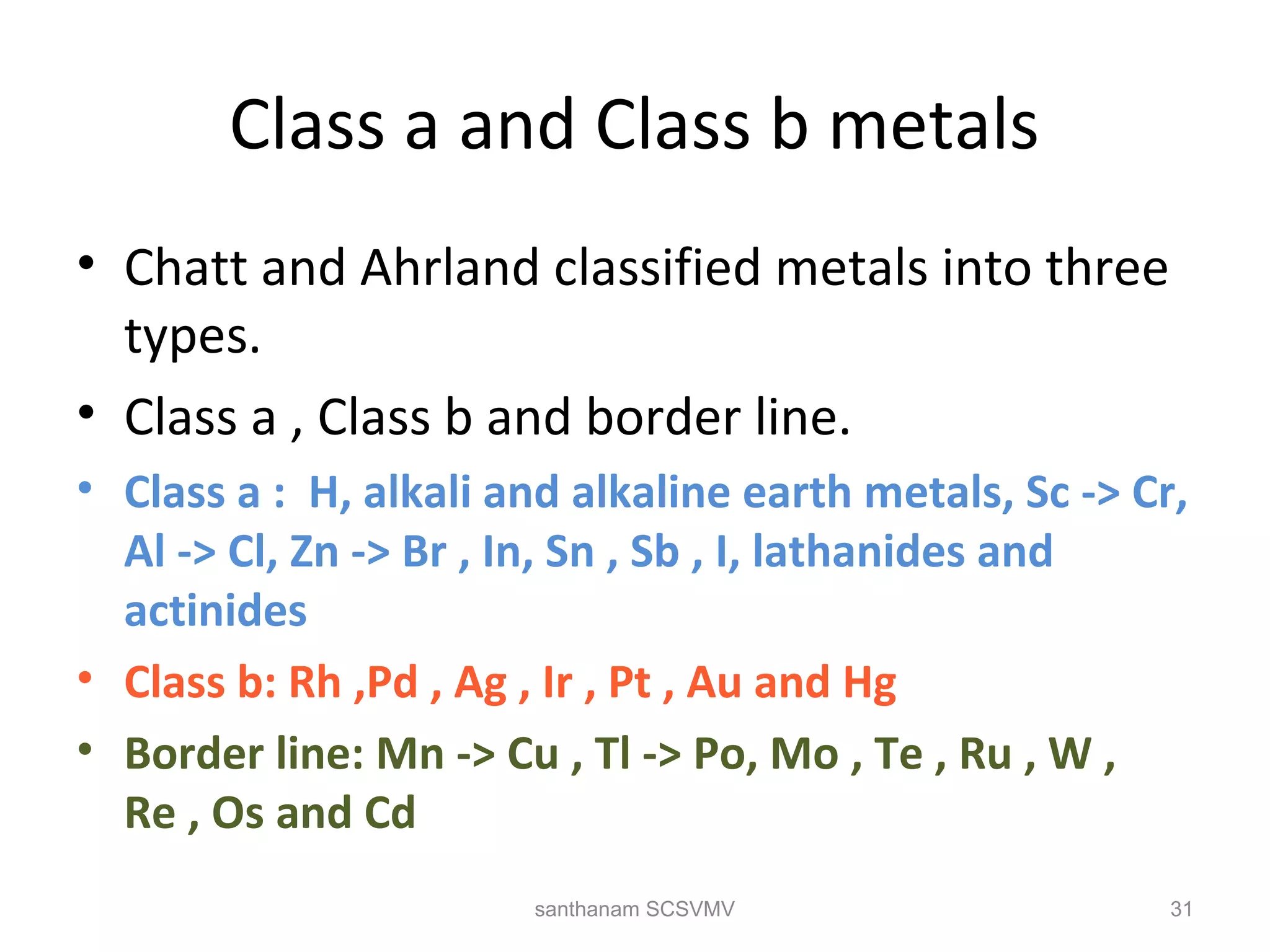
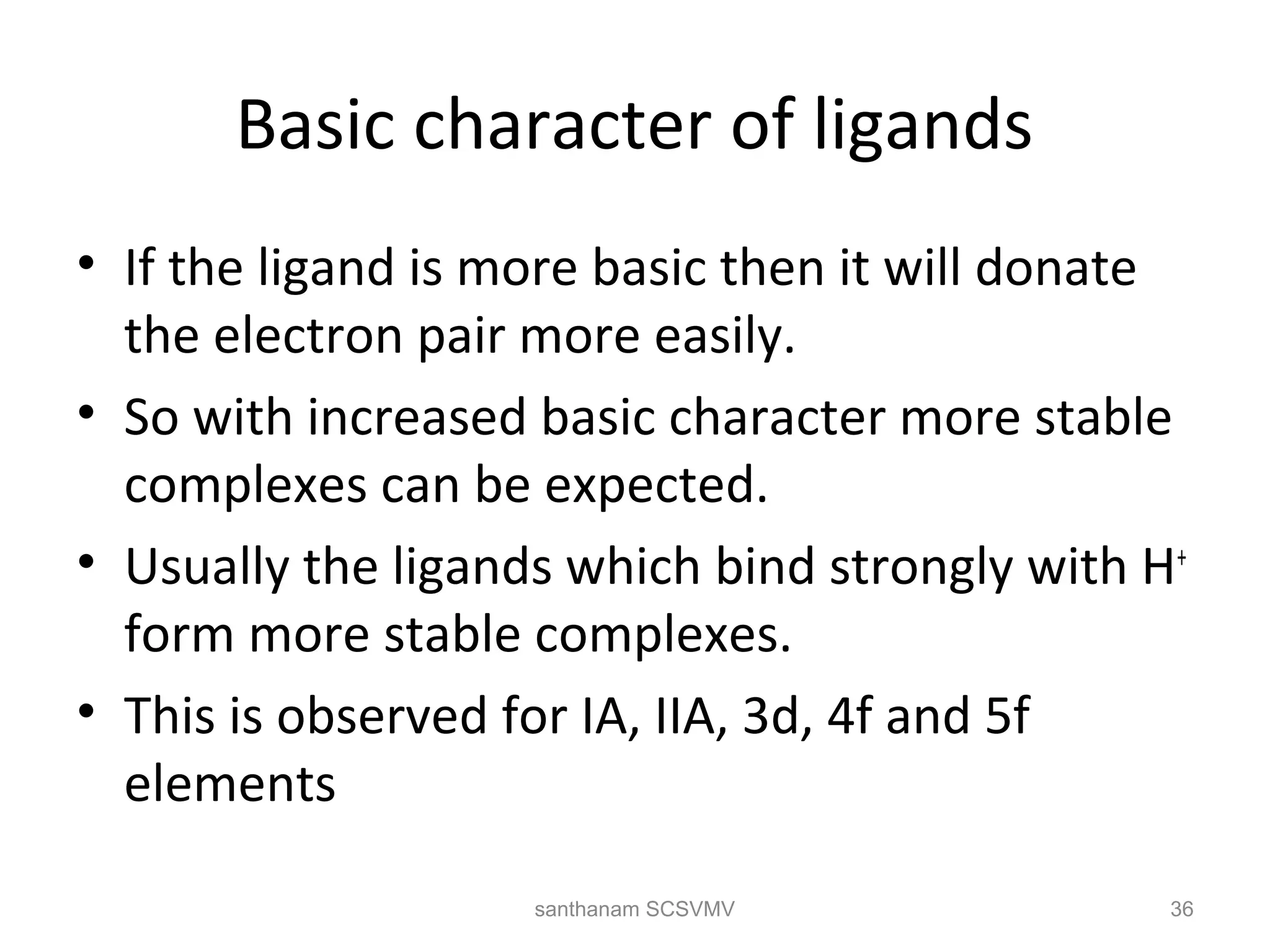
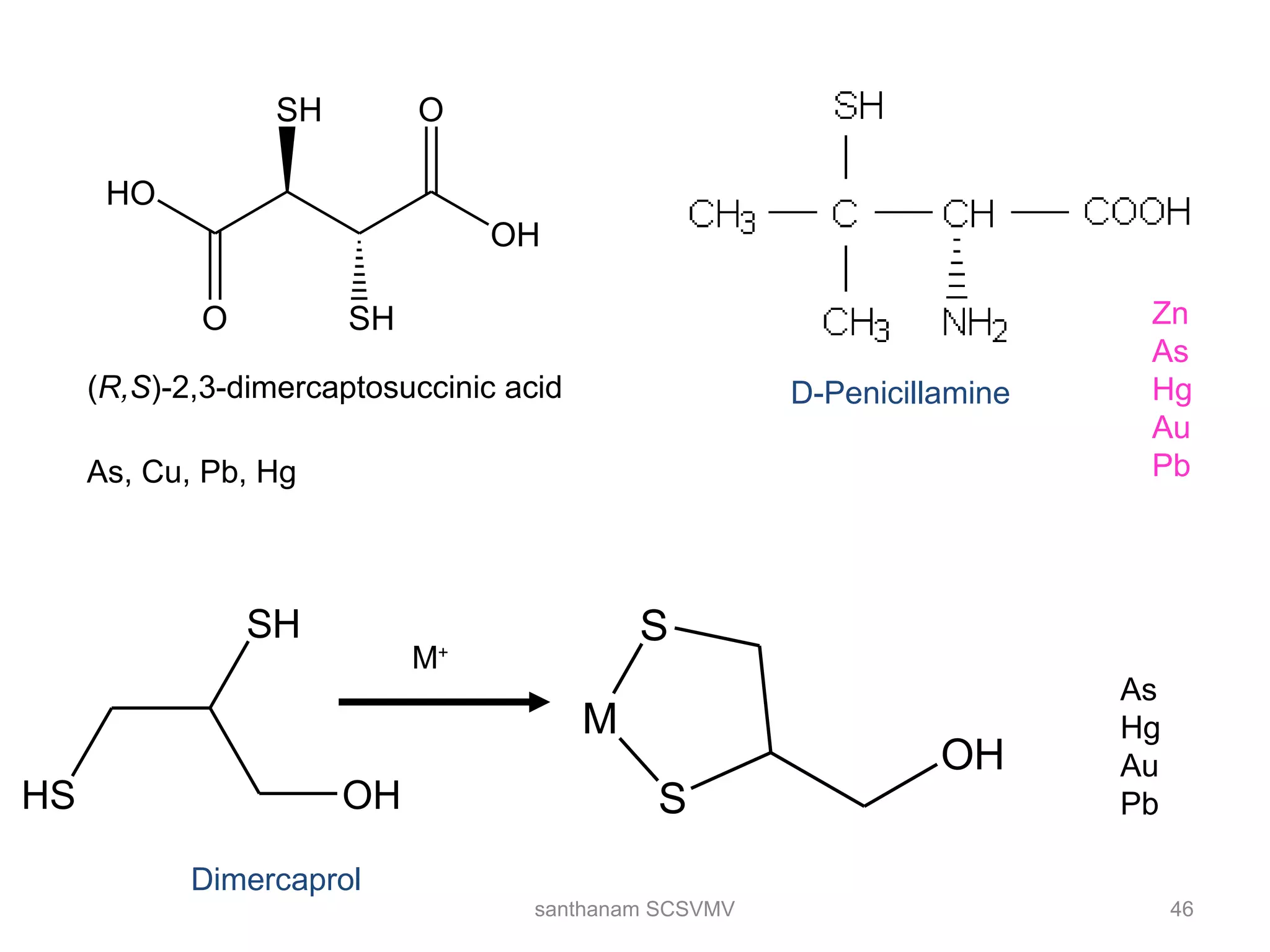
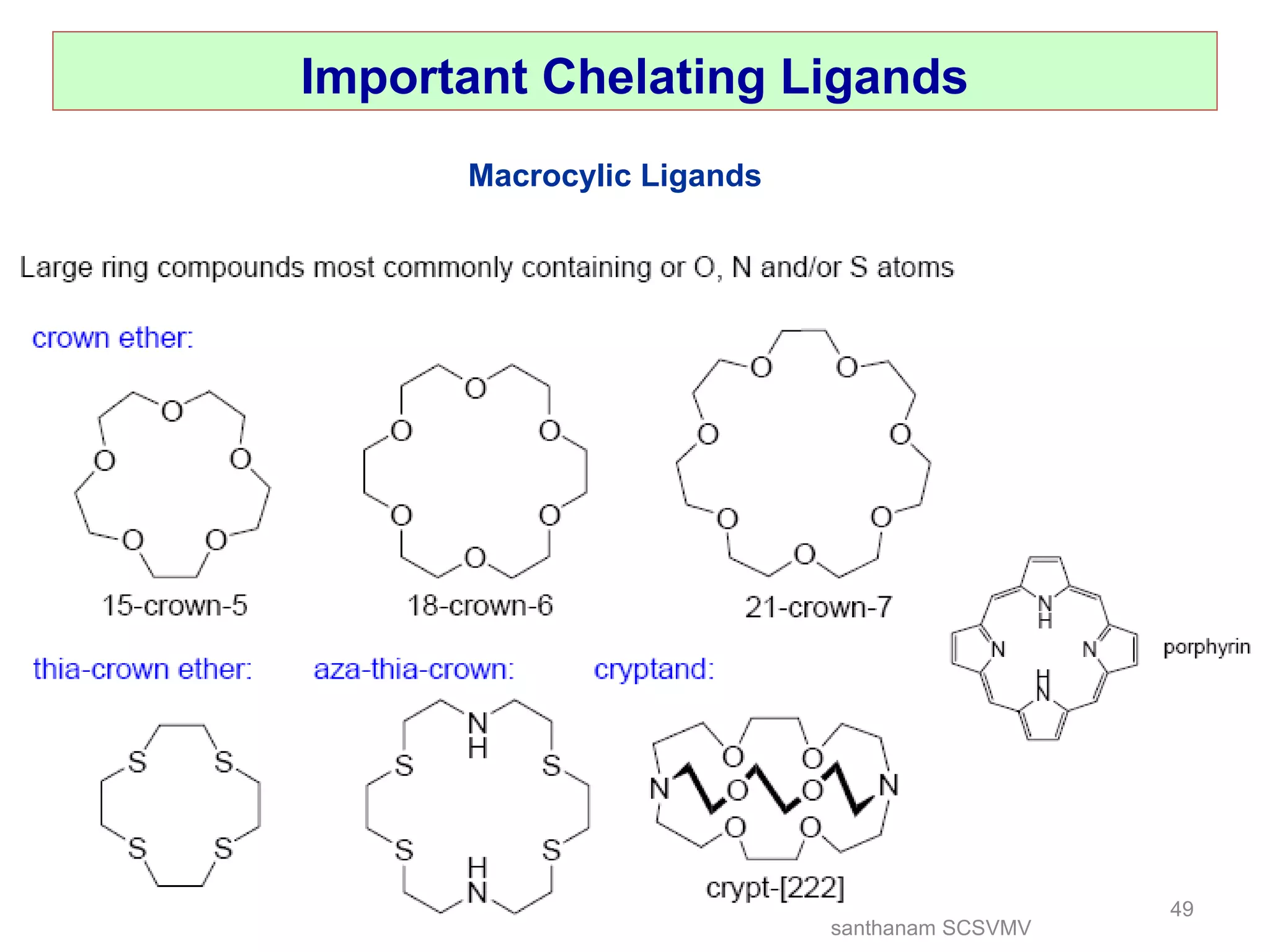
The document discusses various factors that affect the stability of metal complexes. It explains that complexes formed with ligands having higher charge and smaller size are generally more stable. It also discusses the Irving-Williams order of stability and the factors of charge to radius ratio, electronegativity, and basicity of ligands. The chelate effect is described as an important ligand effect where multidentate ligands form more stable complexes due to entropy gains. Kinetic and thermodynamic stability are distinguished from reactivity concepts of labile and inert complexes.





![Stability constant / Formation constant
• According to Bjerrum formation of a complex in
aqueous solution proceeds through a stepwise
fashion with corresponding equilibrium constants
M + L ML K1 = [ML] / [M] [L]
ML + L ML2 K2 = [ML2] / [ML] [L]
ML2 + L ML3 K3 = [ML2] / [ML2] [L]
…………..……………………………….
………….………………………………..
MLn-1 + L MLn Kn = [MLn] / [MLn-1] [L]
These K1,K2 K3 … Kn are called stepwise formation constants
K1
Kn
K3
K2
6santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-6-2048.jpg)
![Overall stability constant
• If the complex formation is considered as a
single step process
M + nL MLn
= [MLn] / [M] [L]ᵝn
7santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-7-2048.jpg)
![Trends in stability constants
[Cu(OH2)4]2+
+ NH3 [Cu(OH2)3(NH3)]2+
+ H2O log K1 = 4.22
[Cu(OH2)3(NH3)]2+
+ NH3 [Cu(OH2)2(NH3)2]2+
+ H2O log K2 = 3.50
[Cu(OH2)2(NH3)2]2+
+ NH3 [Cu(OH2)(NH3)3]2+
+ H2O log K3 = 2.92
[Cu(OH2)(NH3)3]2+
+ NH3 [Cu(NH3)4]2+
+ H2O log K4 = 2.18
• Generally the stepwise stability constant values decrease with
successive replacement by the ligands
8santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-8-2048.jpg)


![Relationship between Kn and ᵝn
• Let us consider
ᵝ3 = [ML3] / [M] [L]3
= [ML3] . [ML2] . [ML]
[M] [L]3
. [ML2] . [ML]
= [ML] . [ML2] . [ML3]
[M] [L] [ML] [L] [ML2] [L]
= K1 . K2 . K3
In general
ᵝn = K .K .K . ….. K
11santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-11-2048.jpg)

![Exemplification
• The above said fact is clearly shown by the
complex [Hg(CN)4]2-
.
Hg2+
+ 4CN-
[Hg(CN)4]2-
ᵝ ≈ 10 42
• The over all formation constant is having very
high value which means that equilibrium is
lying far too right.
13santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-13-2048.jpg)









![Effect of ionic radius
Complex ion Charge on the
ion
Ionic radii (Aₒ
)
Value of ᵝ stability
[BeII
(OH)] +
+2 0.31 107
[MgII
(OH)] +
+2 0.65 120
[CaII
(OH)] +
+2 0.99 30
[BaII
(OH)] +
+2 1.35 4
24santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-24-2048.jpg)
![Effect of charge
Complex ion Charge on the
ion
Ionic radii (Aₒ
)
Value of log ᵝ stability
[FeIII
(CN)6] 3-
+3 31.0
[FeIII
(CN)6] 4-
+2 8.3
CoIII
complex +3 high
CoII
complex +2 low
Almost
same
Almost
same
25santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-25-2048.jpg)







![What are the implications of the following results?
NiCl2 + 6H2O → [Ni(H2O)6]+2
[Ni(H2O)6]+2
+ 6NH3 → [Ni(NH3)6]2+
+ 6H2O log β = 8.6
[Ni(NH3)6]2+
+ 3 NH2CH2CH2NH2 (en)
[Ni(en)3]2+
+ 6NH3
log β = 9.7
[Ni(H2O)6]+2
+ 3 NH2CH2CH2NH2 (en)
[Ni(en)3]2+
+ 6H2O
log β = 18.3
38santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-38-2048.jpg)
![ NH3 is a stronger (better) ligand than
H2O
∆O NH3 > ∆O H2O
[Ni(NH3)6]2+
is more stable
∆G = ∆H - T∆S (∆H -ve, ∆S≈ 0)
∆G for the reaction is negative
Complex Formation: Major Factors
[Ni(H2O)6] + 6NH3
→[Ni(NH3)6]2+
+ 6H2O
39santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-39-2048.jpg)
![Chelate Formation: Major Factors
en and NH3 have similar N-donor environment
but en is bidentate and chelating ligand
rxn proceeds towards right, ∆G negative
∆G = ∆H - T∆S (∆H -ve, ∆S ++ve)
rxn proceeds due to entropy gain
∆S ++ve is the major factor behind chelate
effect
[Ni(NH3)6]2+
+ 3 NH2CH2CH2NH2 (en)
[Ni(en)3]2+
+ 6NH3
40santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-40-2048.jpg)
![Cd2+
+ 4 NH3
↔ [Cd(NH3
)4
]2+
Cd2+
+ 2 en ↔ [Cd(en)2
]2+
Chelate Formation: Entropy Gain
Ligands
4 NH3
4 MeNH2
2 en
∆G
kJmol-1
-42.5
-37.2
-60.7
∆H
kJmol-1
- 53.2
-57.3
-56.5
∆S
JK-1
mol-1
- 35.5
- 67.3
+13.8
log β
7.44
6.52
10.62
Cd2+
+ 4 MeNH2
↔ [Cd(MeNH2
)4
]2+
41santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-41-2048.jpg)
![Reaction of ammonia and en with Cu2+
[Cu(H2O)6]2+
+ en → [Cu(en)(H2O)4]2+
+ 2 H2O
Log K1 = 10.6 ∆H = -54 kJ/mol ∆S = 23 J/K/mol
[Cu(H2O)6]2+
+ 2NH3 → [Cu(NH3)2(H2O)2]2+
+ 2 H2O
Log β2 = 7.7 ∆H = -46 kJ/mol ∆S = -8.4 J/K/mol
Chelate Formation: Entropy Gain
42santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-42-2048.jpg)





![[Cu(OH2)4]2+
+ en [Cu(OH2)2(en)]2+
+ 2 H2O
log K1 = 10.6 ΔH = -54 kJ mol-1
ΔS = 23 J K-1
mol-1
[Cu(OH2)4]2+
+ 2 NH3 [Cu(OH2)2(NH3)2]2+
+ 2H2O
log β2 = 7.7 ΔH = -46 kJ mol-1
ΔS = -8.4 J K-1
mol-1
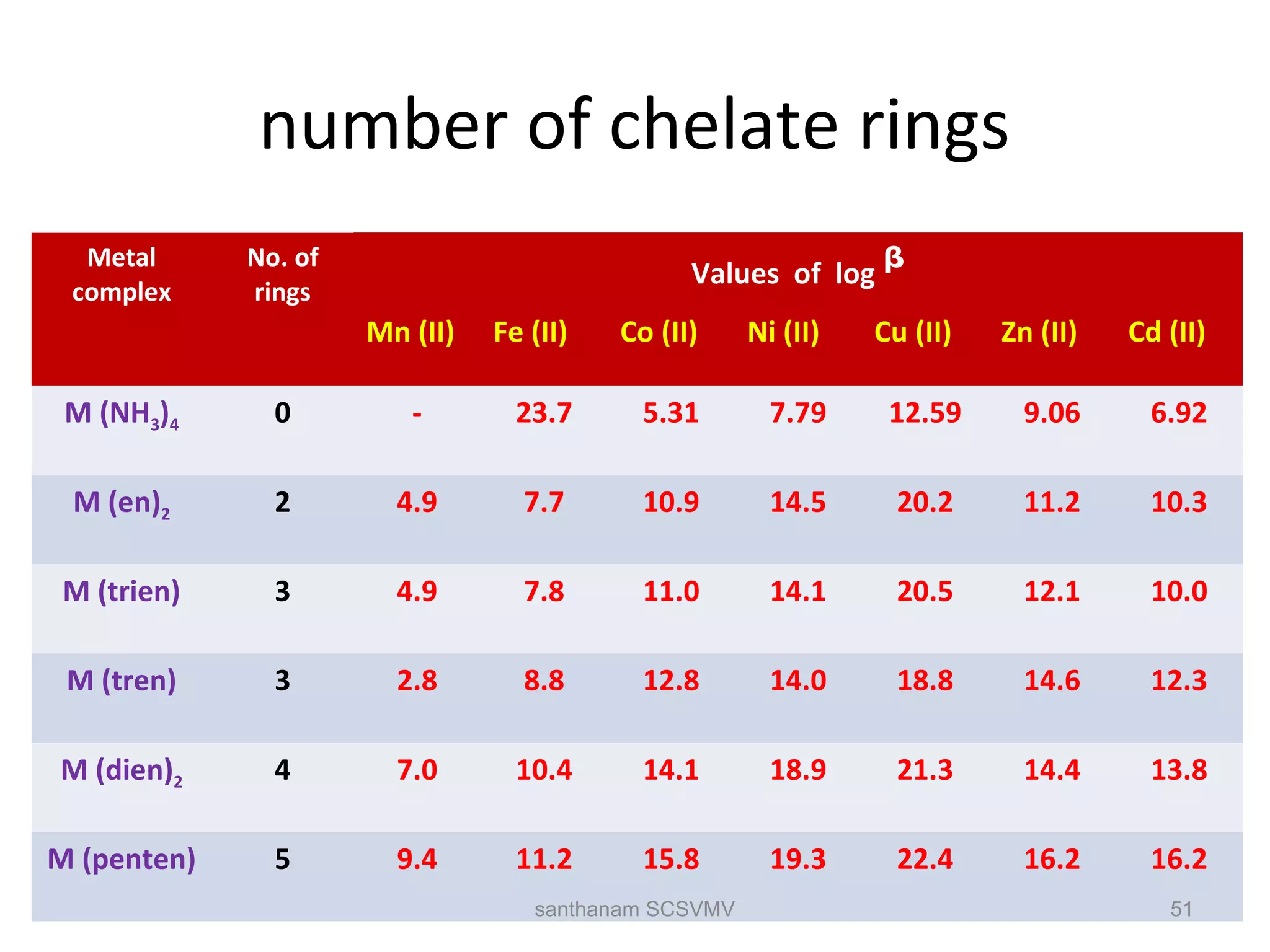
50santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-50-2048.jpg)






![Spectrophotometric method
• While formation of a complex a striking colour change
also occurs.
• The absorption obeys Beer – Lambert’s law
– A = ε . C. l
• A can be measured by using a spectrophotometer
• If ε and l are known then C can be calculated.
• Considering the following reaction,
M2+
+ L ML2+
K = [ML2+
] / [M2+
] [L]
58santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-58-2048.jpg)
![It is known that ,
CM = [M2+
] + [ML2+
]
CL = [L] + [ML2+
]
A = ε [ML2+]. C[ML2+] . l
C[ML2+] = A / ε [ML2+].l
So
[M2+
] = CM - (A / ε [ML2+].l)
[L] = CL - (A / ε [ML2+].l) 59santhanam SCSVMV](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-59-2048.jpg)

![Potentiometric method
• Also known as Bjerrum method
• When ligand is a weak base or acid, there is
competition between hydrogen ions and
metal ions for the ligand .
L + H+
HL+
Ka = [HL+
] / [L] [H+
]
L + M+
ML+
KF = [ML+
] / [L] [M+
]
• If CH,CM and CL are the molar concentrations
santhanam SCSVMV 61](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-61-2048.jpg)
![CH = [H+
] + [HL+
]
CL = [L] + [ML+
] + [HL+
]
CM = [M+
] + [ML+
]
• Solving the equations by using the association
constant of the ligand
[ML+
] = CL-CH+[H+] – CH-[H+
] / Ka [H+
]
[M+
] = CM – [ML+
]
[L] = CH – [H+
] / Ka [H+
]
santhanam SCSVMV 62](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-62-2048.jpg)
![• Except [H+
] all the other parameters are
known , hence the stability constant can be
calculated after measuring the pH of the
solution by using a pH meter
• In order to get precise results the ligand must
be a moderately weak base or acid.
• KF value should be within 105
times of the
association constant
santhanam SCSVMV 63](https://image.slidesharecdn.com/sancomplexstability-161013152714/75/Stability-of-metal-complexes-63-2048.jpg)
