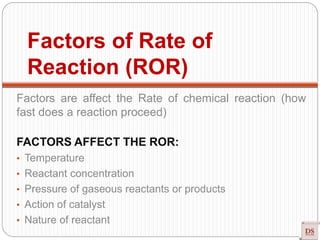
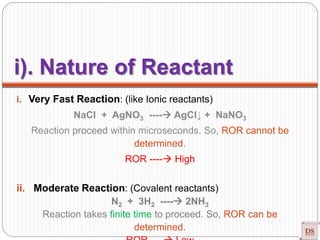
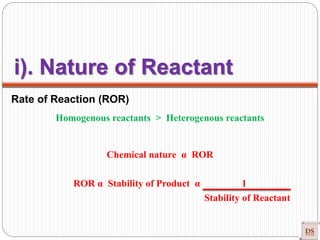
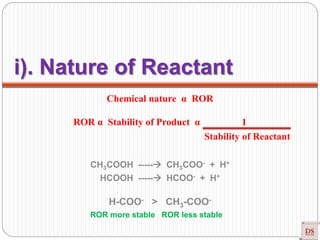
The document provides a comprehensive overview of chemical kinetics, detailing the rate of reaction, factors affecting it, and methods of measuring reaction rates. Key factors include temperature, concentration, pressure, and the presence of catalysts, with various examples illustrating fast, moderate, and slow reactions. The document also discusses rate laws, the order of reactions, and how these variables interact through experimental methods.


![2A + 3B --------- 4C
If, Initial time => t = 0; Conc. a = a0 Conc. = 0
Final time => t = t; Conc. a = at (aFinal - aInitial) Conc. = X
Average ROR =
– ½ [∆A]/∆t = – ⅓ [∆B]/∆t = + ¼ [∆C]/∆t
Average ROR = Rate of change in concentration
Rate of change in time
Average Rate of Reaction
(ROR):](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-9-320.jpg)
![2X + Y --- 3Z
Average ROR =
– ½ [∆X]/∆t = – [∆Y]/∆t = + ⅓ [∆Z]/∆t
Rate of appearance (ROA): Product
ROA of Z = [∆Z]
∆ t
Rate of disappearance (ROD): Reactant
ROD of X = [∆X] ROD of Y = [∆Y]
∆ t ∆ t
Average Rate of Reaction
(ROR):](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-10-320.jpg)
![Example: N2 + 3H2 ------ 2NH3
Average ROR =
– [∆N2]/∆t = – ⅓ [∆H2]/∆t = + ½ [∆NH3]/∆t
Rate of appearance (ROA): Product
ROA of NH3 = [∆NH3]
∆ t
Rate of disappearance (ROD): Reactant
ROD of N2 = [∆N2] ROD of H2 = [∆H2]
∆ t ∆ t
Average Rate of Reaction
(ROR):](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-11-320.jpg)
![Average ROR =
– ½ [∆N2O5]/∆t = + ¼ [∆NO2]/∆t = + [∆ O2]/∆t
Rate of appearance = [∆NO2] = 6Mol/L/s
∆ t
ROR = + ¼ [∆NO2]/∆t = + ¼ ˟ 6 = 1.5 Mol/L/sec
ROR = – ½ [∆N2O5]/∆t
1.5 = – ½ [∆N2O5]/∆t
3 Mol/L/sec = [∆ N2O5]
∆ t
Q. 2N2O5 ----------- 4NO2 + O2
If ROA of NO2 is 6Mol/L/sec. then,
find (i) ROR, and (ii) ROD of N2O5.](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-13-320.jpg)
![2A + 3B --- 4C
Initial time: ∆t = 0
Final time: ∆t = dt
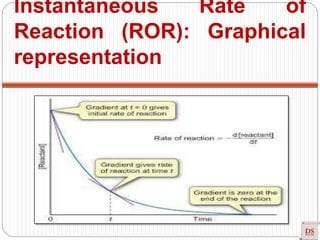
Instantaneous ROR =
– ½ d[A]/ dt = – ⅓ d[B]/ dt = + ¼ d[C]/ dt
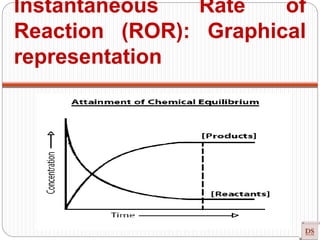
Instantaneous Rate of
Reaction (ROR):](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-14-320.jpg)
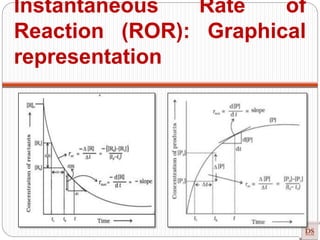
![Slope = tan ɵ = d[A2 –
A1]/d(t2 – t1)
Slope = tan ɵ = d[B2 – B1]/
d(t2 – t1)
2A + 3B --- 4C
Instantaneous ROR =
–½d[A2 – A1]/d(t2 – t1) = – ⅓d[B2 – B1]/d(t2 – t1) = +¼d[C2 – C1]/d(t2 – t1)
Slope = tan ɵ = d[C2 – C1]/
d(t2 – t1)](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-18-320.jpg)
![Slope = tanɵ =
d[NH3]/dt
Slope = tanɵ =
d[O2]/dt
4NH3 + 5O2 -- 4NO + 6H2O
Instantaneous ROR =
–¼d[NH3]/dt = –⅕d[O2]/dt = +¼d[NO]/dt = +⅙d[H2O]/dt
Slope = tanɵ =
d[NO]/dt
Slope = tanɵ =
d[H2O]/dt](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-19-320.jpg)
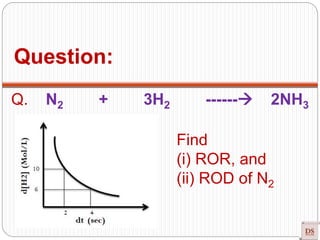
![Q. N2 + 3H2 ------
2NH3
Find
(i) ROR, and
(ii) ROD of N2
Instantaneous ROR =
– d[N2]/ dt = – ⅓ d[H2]/ dt = + ½ d[NH3]/ dt
Slope = tan ɵ = (R2 – R1) = (6 - 10) = -2
(t2 – t1) (4 – 2)
ROR = – ⅓ d[H2]/dt
ROR = - ⅓ ˟ -2 => 2/3 Mol/L/sec
ROD = d[N2] = - d[N2] => 2/3 Mol/L/sec
dt dt](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-21-320.jpg)
![Rate of Reaction (ROR) ----- (mostly in
Solid state)
Surface area of a solid reactant More area for
reactants to be in contact
ROR α Surface area of reactant
Eg: Sugar powder/solute (small molecules)
easily dissolve in milk/solvent/water than
sugar cubes. [Due to more surface area of
molecules].
iii). Surface area of
Reactant](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-27-320.jpg)
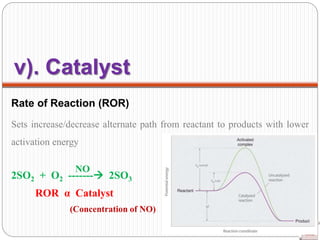

![2A + B ------ 2C
Reactants Product
r = K [A]1 [B]2
Experimental method (ROR):
If, [A] ---- Double ROR ------- 2 times increase
ROR α [A]1
If, [B] ---- Double ROR ------- 4 times increase
ROR α [B]2
[⁖ ROR = r]
Rate Law of Expression
⁖ where as, K = Rate constant; [A] = Concentration of A reactant; [B] =
Concentration of B reactant; ROR = Rate of reaction; r = Rate law.](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-35-320.jpg)
![2A + B ------ 2C
Reactants Product
r = K [A]2 [B]1 This method followed
in only single step
reactions.
Guldberg & Wagge method:
If, [A] ---- Double ROR ------- 4 times increase
ROR α [A]2
If, [B] ---- Double ROR ------- 2 times increase
ROR α [B]1
[⁖ ROR = r]
Law of Mass Action
(as according stoichiometric coefficient)
⁖ where as, K = Rate constant; [A] = Concentration of A reactant; [B] = Concentration
of B reactant; ROR = Rate of reaction; r = Rate law.](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-36-320.jpg)
![2A + B ------ 2C
Reactants Product
r = K [A]x [B]y
Experimental method (ROR):
r α [A]x
r α [B]y
Rate Law of Expression
⁖ where as, K = Rate constant; [A] = Concentration of A reactant (Mol/Lit); [B] =
Concentration of B reactant (Mol/Lit); r = Rate law/ Rate of reaction; x = Order of
reaction with respect to A; y = Order of reaction with respect to B.](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-37-320.jpg)
![2A + B ------ 2C
Reactants Product
Rate Law --- by experimental method (ROR):
K = Rate constant/ velocity constant/ specific reaction rate (per unit)
r α [A]x [B]y
If, concentration of all reactants or products in rate law expression is unity (1)
[A] = [B] = 1
K = Specific reaction rate (per unit)
r = K [A]x [B]y
r = K](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-39-320.jpg)
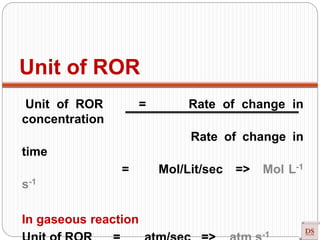
![Unit of Rate Constant (K)
Unit of ROR = Mol/Lit/sec => Mol L-1 s-1
ROR = K [A]x [B]y
K = ROR
[A]x [B]y
Unit of K = Mol/Lit (⁖ x + y =
n)
Sec
[Mol/Lit]x [Mol/Lit]y
= [Mol/Lit/sec] => [Mol/Lit]1-n => [Mol/Lit]1-n](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-40-320.jpg)
![Unit of Order of Reaction
(n) & Rate Constant (K)
Order of Reaction (n) Unit of K [Mol/Lit]1-n /sec]
Zero order of reaction [Mol/Lit] /sec
First order of reaction sec-1
Second order of reaction [Mol/Lit]-1 /sec
Half order of reaction [Mol/Lit]3/2 /sec](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-41-320.jpg)
![K = 7 ˟ 10-4 Lit2 Mol-2 sec-1
[Mol/Lit]1-n /sec = [Mol/Lit]-2 /sec
1 – n = -2
Third order of reaction
Q. If rate constant is 7 ˟ 10-4 Lit2 Mol-2 sec-
1 of any chemical reaction. Find order of
reaction?](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-42-320.jpg)
![Q. 2A + 3B ----- 4C
If order of reaction with respect to A & B is 2 & -1. Write rate law
expression and calculate order of reaction? What is the effect on rate;
when,
(i). Concentration of A is doubled alone
(ii). Concentration of B is halved alone
(iii). Concentration of A & B is doubled
(iv). Volume of container increases 3 times
r = K [A]x [B]y
r = K [A]2 [B]-1
n = x + y
n = 2 + (-1) = 1 (First order of reaction)
K = sec-1
(i). r = K [A]2 [B]-1
r’ = K [2A]2 [B]-1
r’ = K [2A]2 [B]-1 => r’ = 22 => r’ = 4r
r K [A]2 [B]-1 r
Rate increases 4 times](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-43-320.jpg)
![Q. 2A + 3B ----- 4C
If order of reaction with respect to A & B is 2 & -1. Write rate law
expression and calculate order of reaction? What is the effect on rate;
when,
(i). Concentration of A is doubled alone
(ii). Concentration of B is halved alone
(iii). Concentration of A & B is doubled
(iv). Volume of container increases 3 times
(ii). r = K [A]2 [B]-1
r’ = K [A]2 [B/2]-1
r’ = K [2A]2 [B/2]-1 => r’ = 1 -1 => r’ = 2
r K [A]2 [B]-1 r 2 r
Rate increases 2 times
(iii). r = K [A]2 [B]-1
r’ = K [2A]2 [2B]-1
r’ = K [2A]2 [2B]-1 => r’ = (2)2 ˟ (2)-1 => r’ = 4 = 2
r K [A]2 [B]-1 r r 2
Rate increases 2 times](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-44-320.jpg)
![Q. 2A + 3B ----- 4C
If order of reaction with respect to A & B is 2 & -1. Write rate law
expression and calculate order of reaction? What is the effect on rate;
when,
(i). Concentration of A is doubled alone
(ii). Concentration of B is halved alone
(iii). Concentration of A & B is doubled
(iv). Volume of container increases 3 times
(iv). r = K [A]2 [B]-1
Volume α 1/Concentration
[A] = Number of Moles
Volume of Solution (in Lit)
Volume increase 3 times --- Concentration decreases 3 times
r = K [A]2 [B]-1
r’ = K [A/3]2 [B/3]-1
r’ = K [A/3]2 [B/3]-1 => r’ = [1/3]2 [1/3]-1 => r’ = 3
r K [A]2 [B]-1 r r 9
Rate decreases 3 times
(Due to number of moles per volume
=> Molarity)](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-45-320.jpg)
![Note:
1. Stoichiometric coefficients having nothing to do with order
2N2O5 -------- 4NO2 + O2
By Expt.: r = K [N2O5]1
2. In Rate law, concentration terms of products may be present
O3 --------- O2
By Expt.: r = K [O3]2 [O2]-1
3. In Rate law, concentration terms of some reactant may be present
NO2 + CO -------- NO + CO2
By Expt.: r = K [NO2]2 [CO]0 => r = K
[NO2]2](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-46-320.jpg)
![Note:
4. In Rate law expression, concentration term of catalyst may be present.
Catalyst ---- depend on reactant/product of reaction
2SO2 + O2 -------- 4SO3
By Expt.: r = K [O2]2 [NO]1
NO](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-47-320.jpg)
![Simple/ Elementary single
step reaction:
At slowest (single) step, -- Rate of Reaction -- Rate determined
3A + 2B -- C
Step1: 2A + B -- P
Step2: P + A -- Q
Step3: Q + B -- C
Final reaction: 3A + 2B -- C
By Expt.: r = K [A]2 [B]1
Example:
H2 + I2 ----- 2HI
r = K [H2]1 [I2]1
Single step Reaction
Guldbery & Wagge
Method
Law of Mass Action](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-48-320.jpg)
![Simple/ Elementary single
step reaction:
At slowest (single) step, -- Rate of Reaction -- Rate determined
3A + 2B -- C
Step1: 2A + B -- P
Step2: P + A -- Q
Step3: Q + B -- C
Final reaction: 3A + 2B -- C
By Expt.: r = K [A]2 [B]1
Example:
H2 + I2 ----- 2HI
r = K [H2]1 [I2]1
By Expt.: It’s not single step reaction
Single step Reaction
Guldbery & Wagge
Method
Law of Mass Action](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-49-320.jpg)
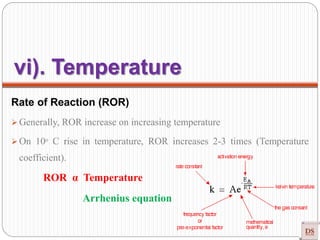
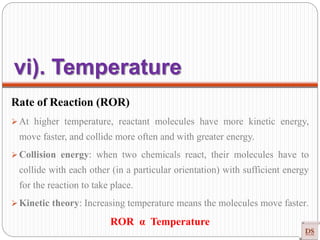
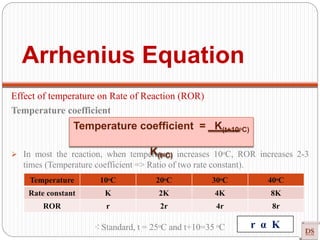
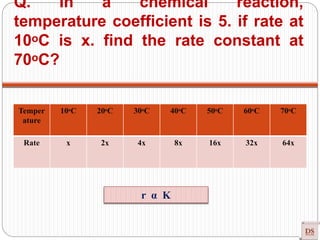
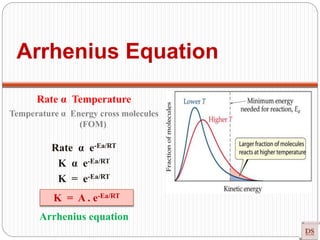
![Arrhenius Equation
Effect of temperature on Rate of Reaction (ROR)
Rate α Temperature
Rate = K [conc]n
Generally, ROR increases on increasing temperature (Approximately their
dependency of K on T)
In most the reaction, when temperature increases 10ᵒC, ROR increases 2-3
times (Temperature coefficient).
r = K [A]x [B]y
r α K](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-50-320.jpg)
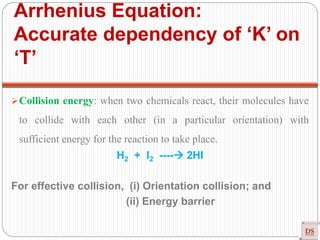
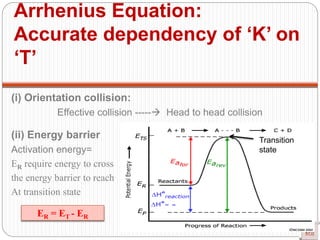
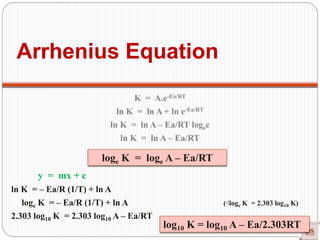
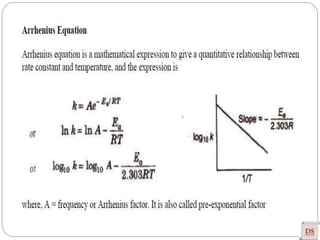
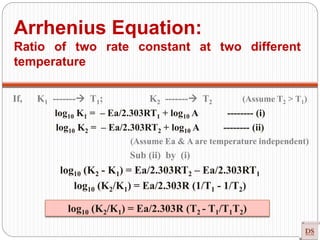
![Arrhenius Equation:
Accurate dependency of ‘K’ on
‘T’
Arrhenius equation
[R = Universal gas constant = 8.314 joule/mole K]
Exponentially Increases
Rateconstant(K)
Temperature
(Joule/mole)
(constant)
(universal
constant)
(Kelvin)](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-53-320.jpg)
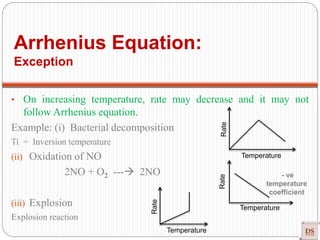
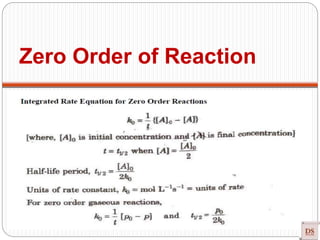
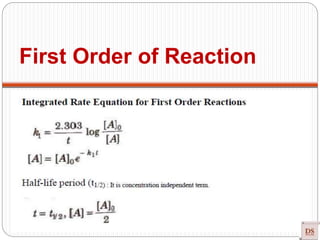
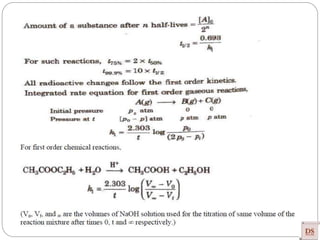
![Single step reaction
2A + 2B -------- 3AB
A-------A B--------B a------b
•There is no intermediate
•Activation energy:
Rate law: r = K [A] [B]
Experimentally method
Guldberg and Wagge method : Law of mass action
Single step reaction](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-66-320.jpg)
![1. H2 + I2 ------ 2HI
Reactant ------ Product
A ----- PRODUCT
Molecularity = 1 (Molecules in reactant)
r = K [A]
2. A + B ------ > PRODUCT
Molecularity = 2
r = K [A] [B]
Single step reaction](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-67-320.jpg)
![3. A + 2B ---- Product
Molecularity = 3
r = K [A] [B]
Maximum molecularity ===== single step reaction
• Simultaneous collision
• Proper collision
• Cross energy barrier
Single step reaction](https://image.slidesharecdn.com/chemicalkinetics-200608073620/85/Chemical-Kinetics-68-320.jpg)