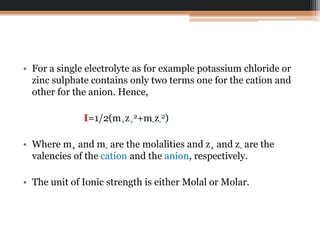
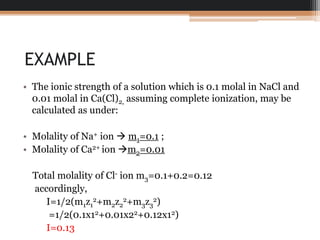
The document explains the principle of ionic strength, which quantifies the electrical intensity of ions in a solution using a specific formula involving molalities and valencies of the ions. It provides a calculation example for a solution containing NaCl and Ca(Cl)2 to illustrate how to determine ionic strength. The unit of ionic strength can be expressed in either molal or molar terms.