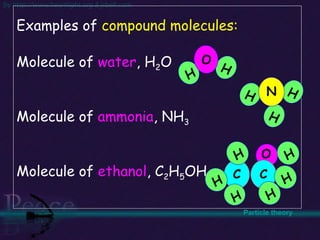
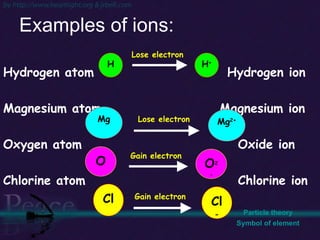
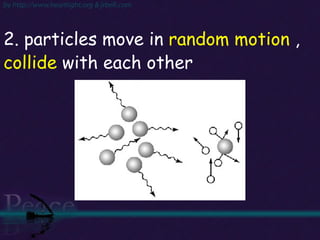
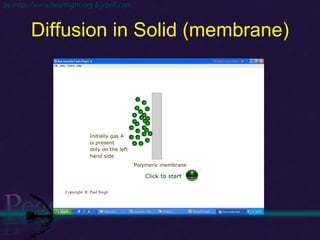
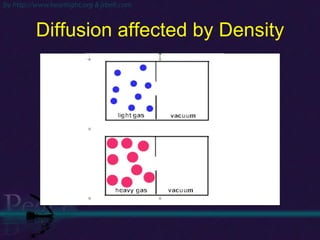
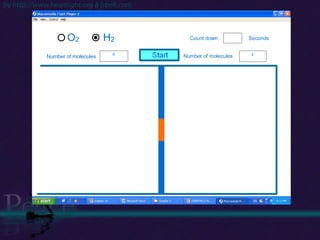
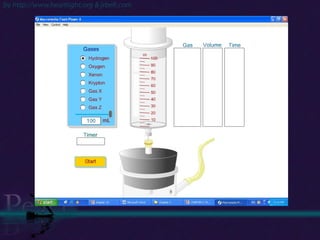
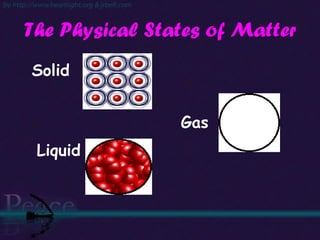
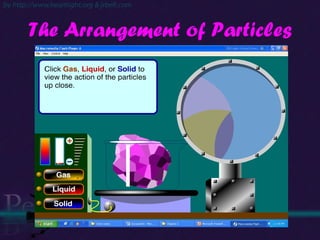
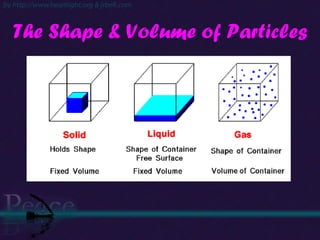
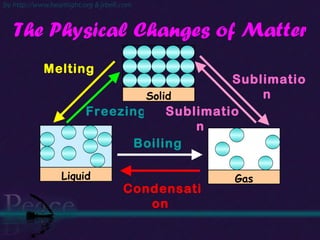
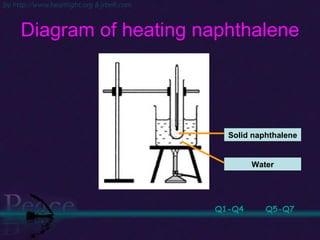
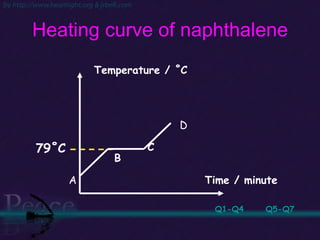
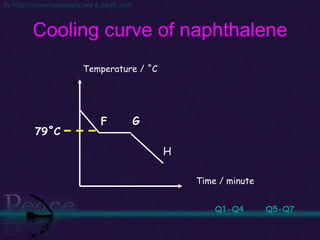
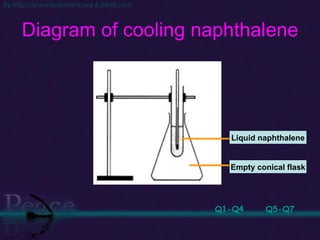
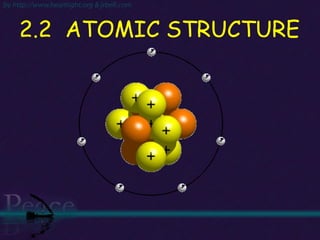
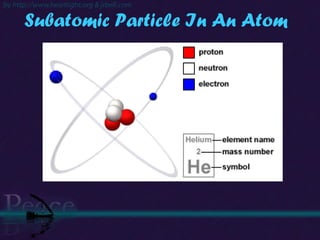
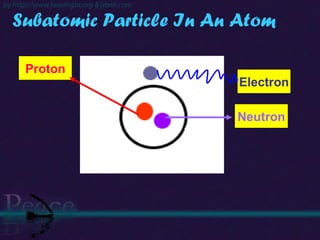
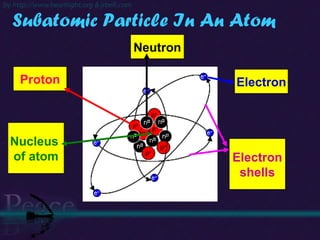
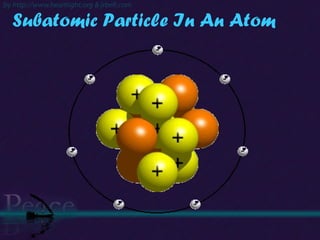
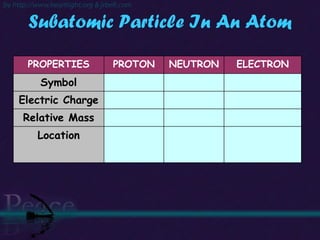
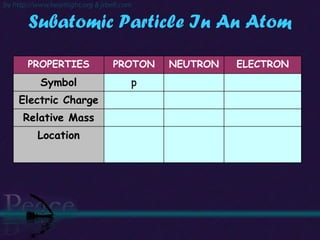
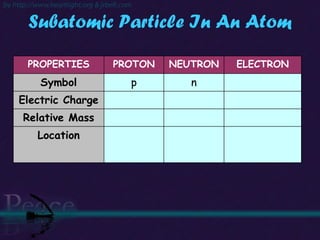
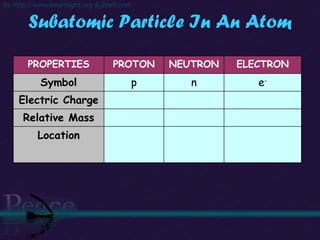
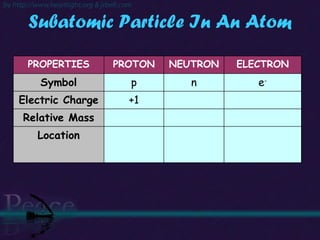
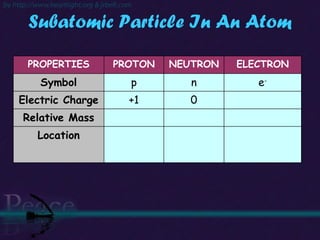
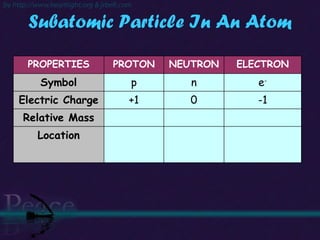
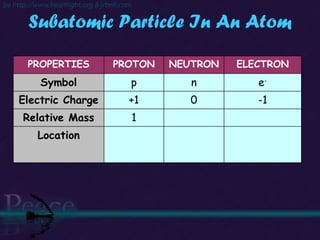
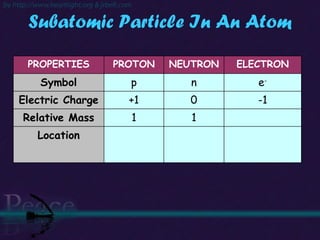
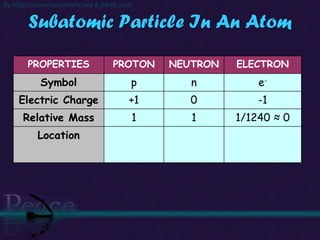
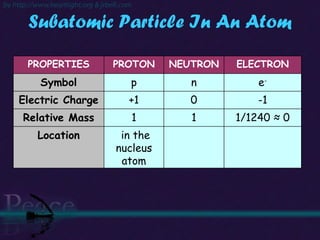
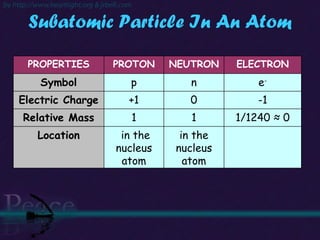
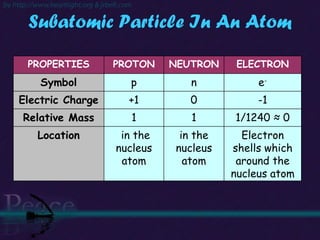
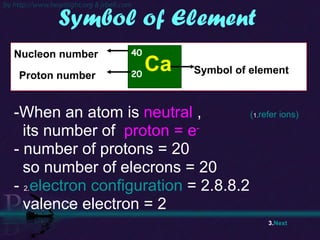
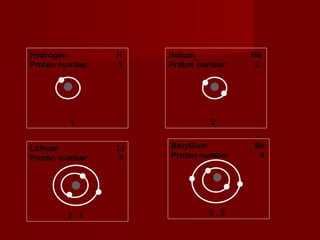
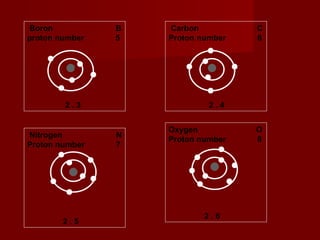
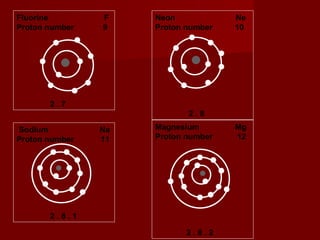
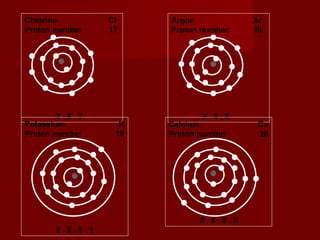
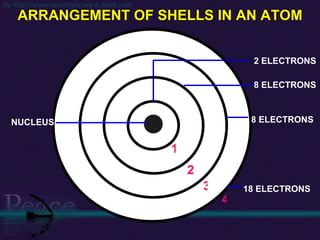
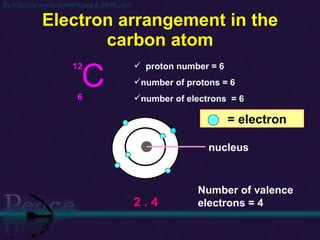
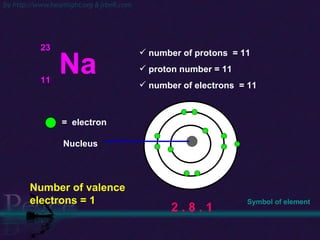
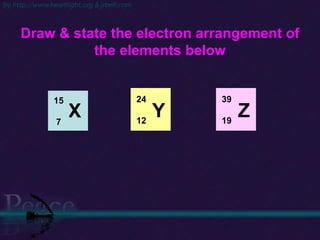
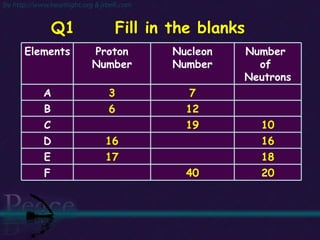
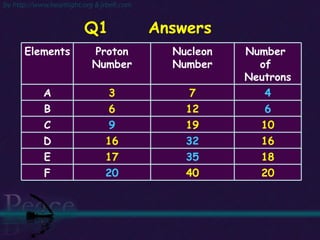
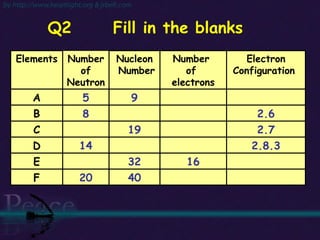
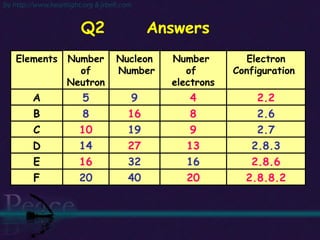
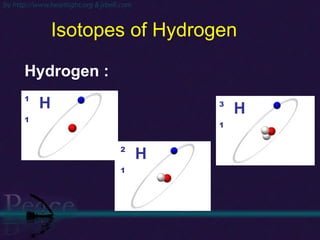
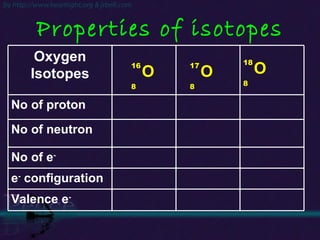
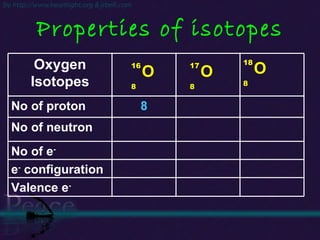
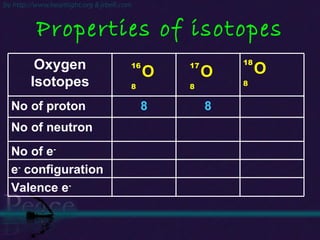
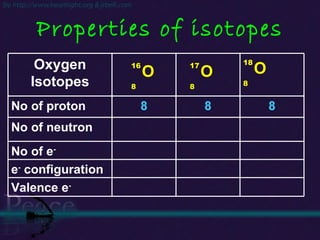
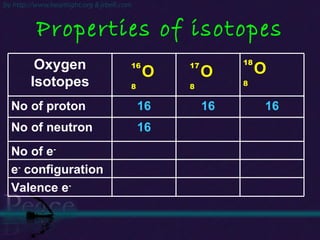
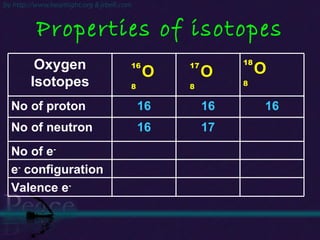
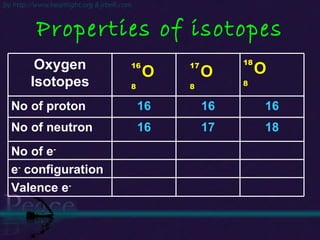
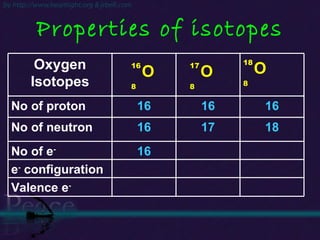
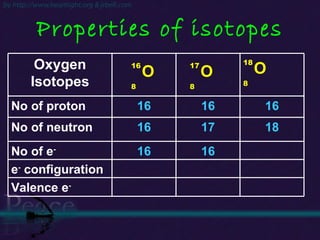
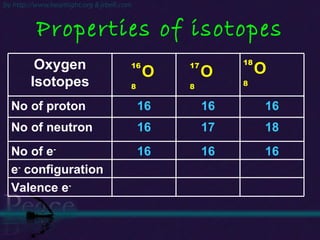
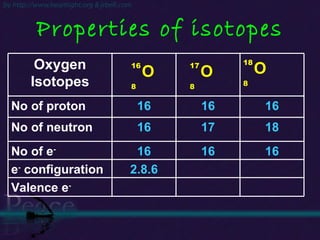
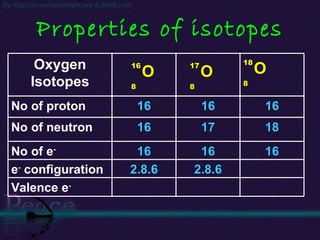
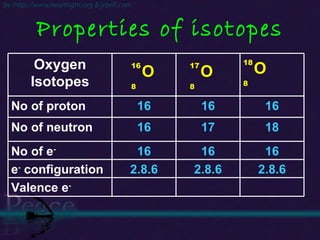
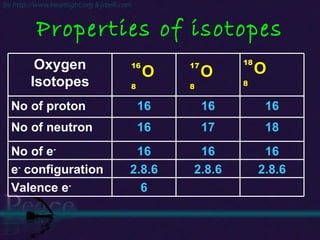
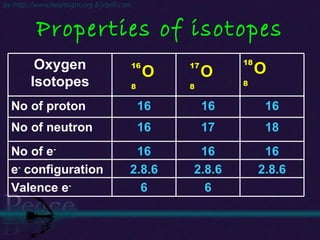
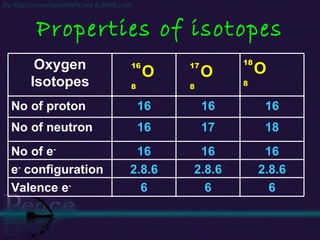
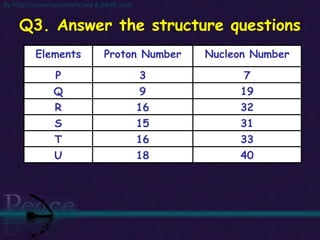
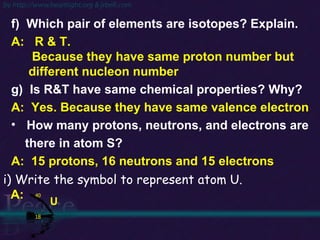
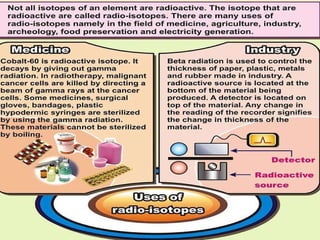
The document discusses the structure of atoms and isotopes. It begins by defining matter and the particle theory of matter. It then explains that atoms are made up of protons, neutrons and electrons. The atomic structure of various elements is discussed through their electron configurations. Isotopes are then introduced as atoms of the same element that have the same number of protons but different numbers of neutrons. Examples of isotopes including hydrogen and oxygen isotopes are provided.