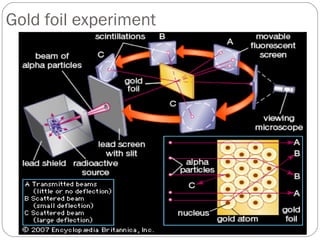
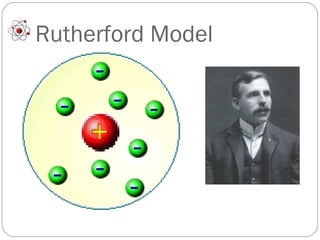
Dalton's atomic theory proposed that matter is made of small indivisible particles called atoms, atoms of the same element are identical, and atoms of different elements have different masses and properties. Rutherford discovered the nucleus of the atom through his gold foil experiment, finding that some alpha particles bounced off the small, dense nucleus at the center of the atom. Chadwick discovered the neutron through experiments with excessively charged atoms, an uncharged particle that helped explain nuclear structure.