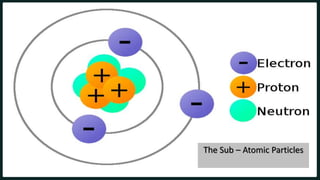
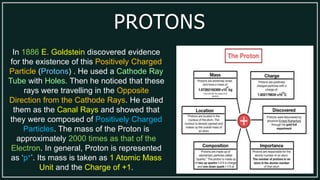
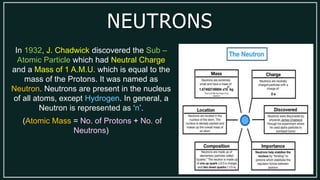
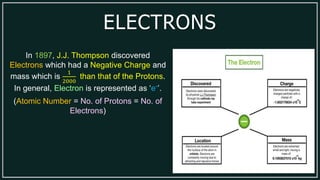
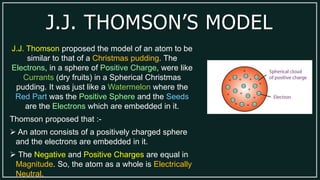
1) An atom is made up of protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, while electrons orbit the nucleus.
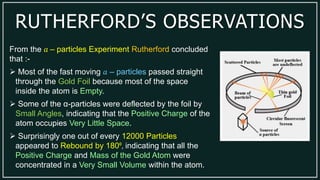
2) Rutherford's gold foil experiment showed that the mass and positive charge of an atom are concentrated in a small nucleus.
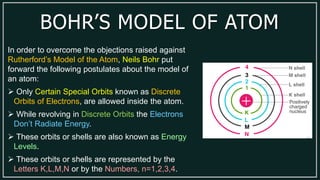
3) Bohr's model improved upon Rutherford's by proposing that electrons can only orbit in discrete, fixed energy levels rather than any path, resolving the issue of electrons radiating energy in orbits.