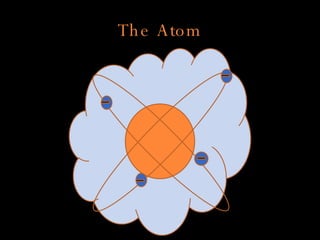
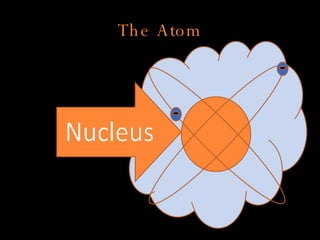
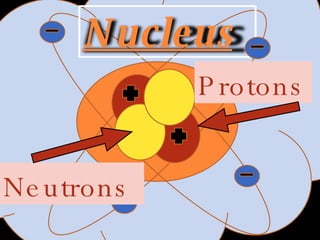
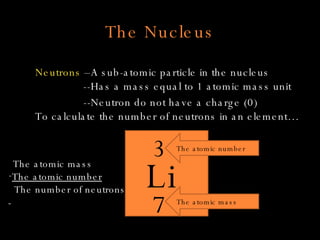
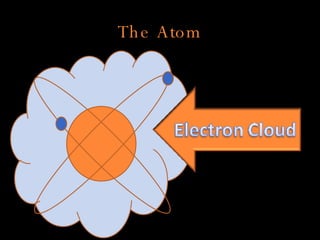
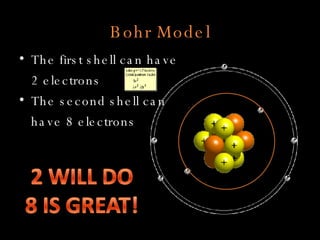
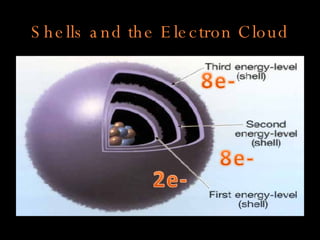
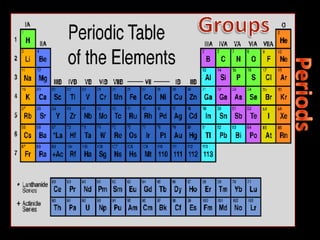
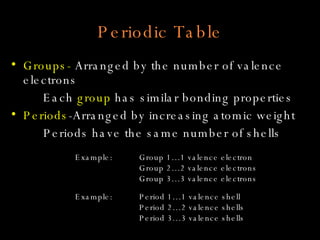
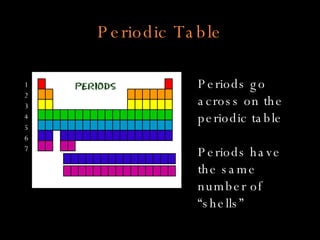
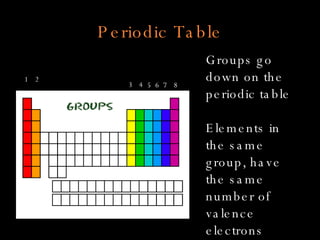
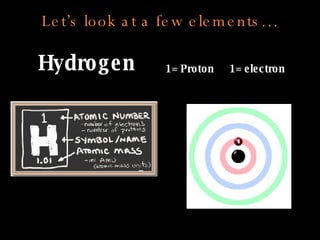
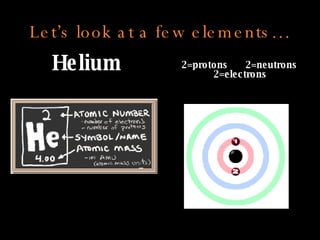
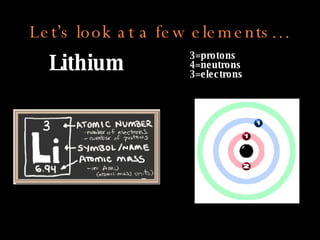
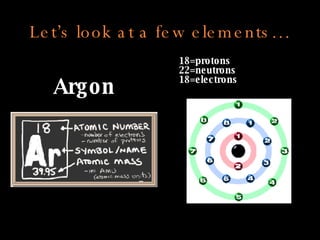
The document defines key atomic terms like atom, nucleus, protons, neutrons, and electrons. It describes how atoms are made up of a tiny nucleus surrounded by an electron cloud. Atoms of the same element have the same number of protons. The number of neutrons and electrons can vary. The periodic table arranges elements in periods and groups based on their atomic structure and number of valence electrons.