1. Elements are the basic building blocks of all matter and are made up of atoms, which are the smallest particles of an element that retain its chemical properties.
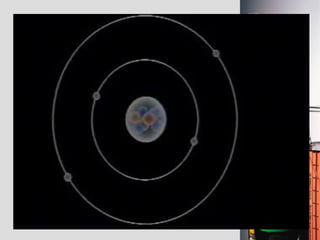
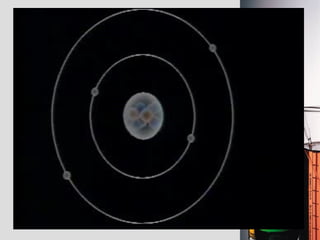
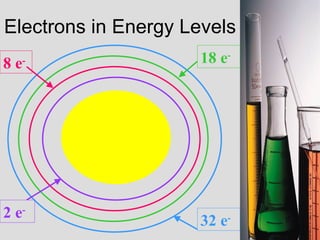
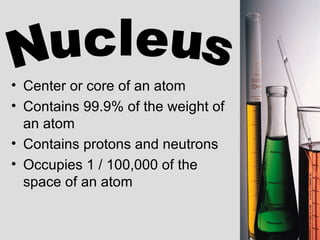
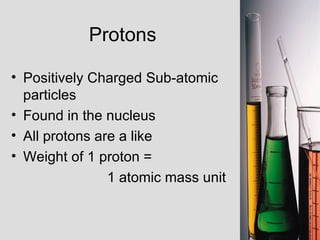
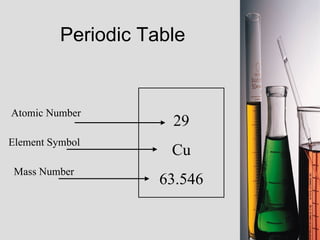
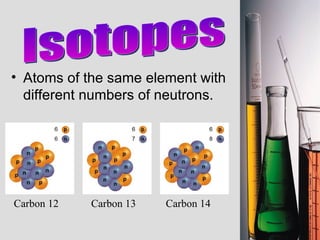
2. Atoms consist of a tiny, dense nucleus surrounded by electrons. The nucleus contains protons and neutrons, while electrons orbit the nucleus. The number of protons determines the element.
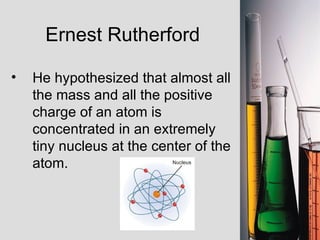
3. Modern atomic theory developed through the works of scientists like Dalton, Thomson, Rutherford, and Bohr, who discovered the subatomic particles and proposed models of atomic structure.