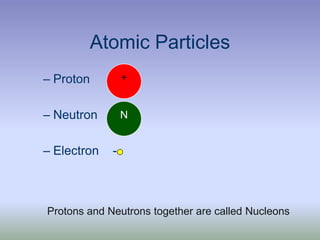
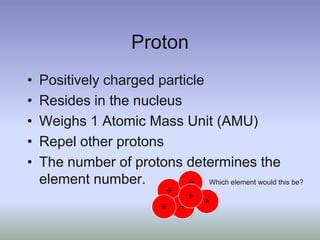
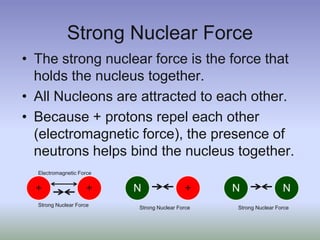
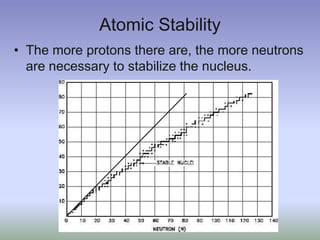
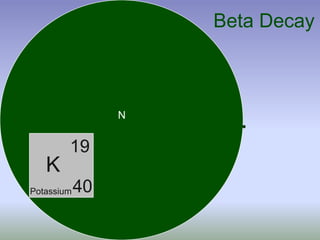
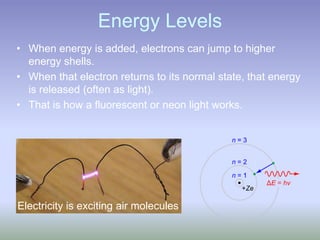
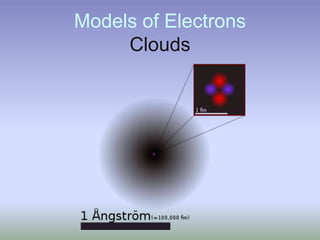
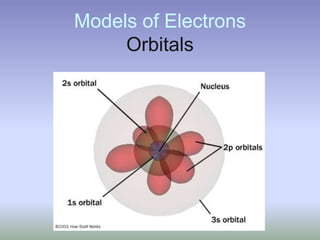
This document summarizes atomic structure and particles. It discusses protons, neutrons, and electrons, and how they make up the nucleus and electron cloud of an atom. Protons determine the element, while neutrons and atomic mass take both protons and neutrons into account. The nucleus is held together by the strong nuclear force between nucleons, while electrons orbit outside the nucleus and usually equal the number of protons. Isotopes of an element have different numbers of neutrons.