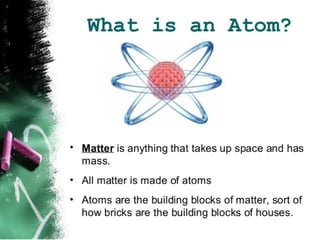
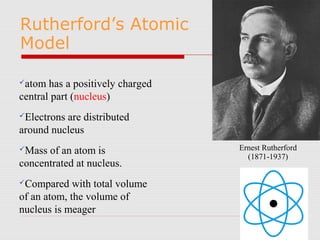
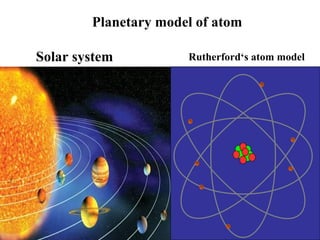
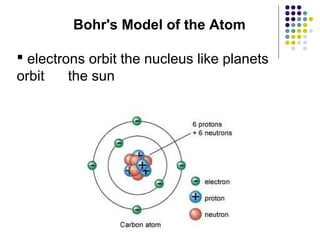
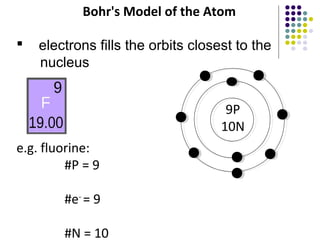
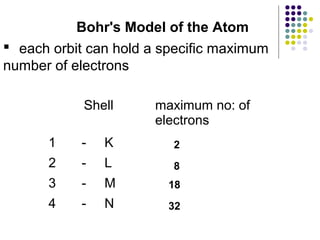
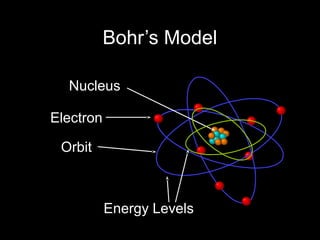
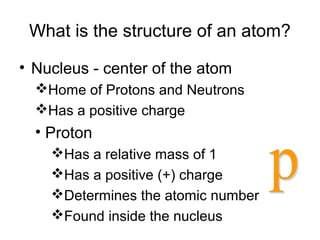
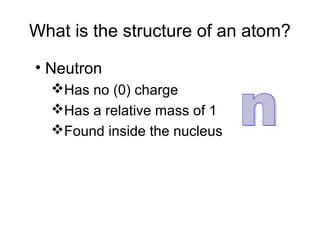
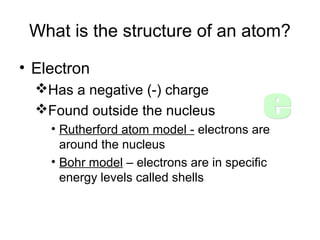
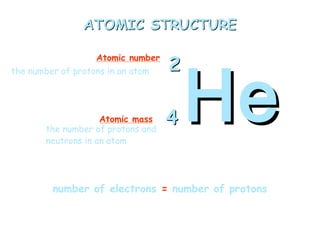
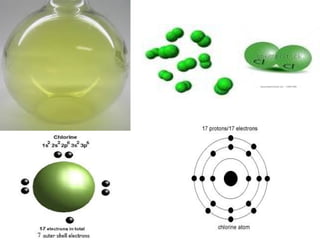
Atoms are the basic building blocks of all matter. John Dalton proposed that atoms are indivisible and identical for each element. Rutherford discovered that atoms have a small, dense nucleus at the center surrounded by electrons. Niels Bohr modeled atoms with electrons orbiting the nucleus in fixed energy levels or shells. The nucleus contains protons and neutrons, while electrons orbit outside. The number of protons determines the element, and protons and neutrons together equal the mass number.