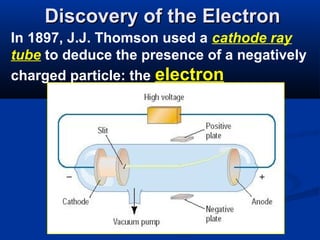
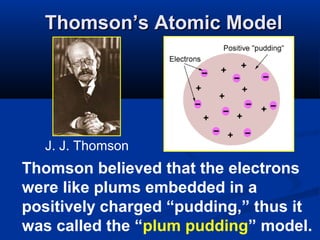
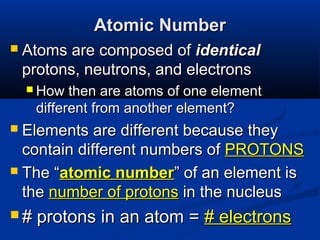
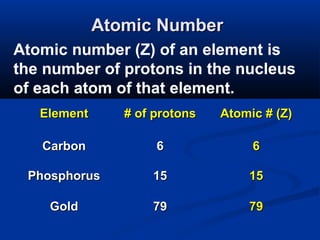
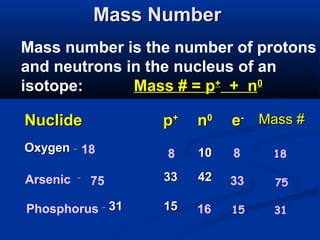
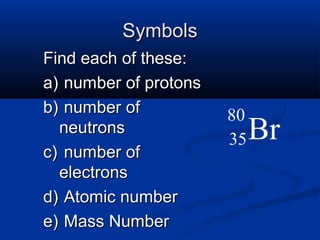
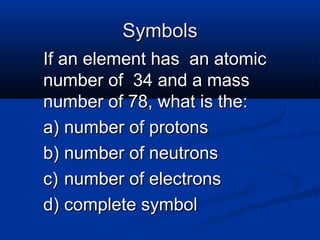
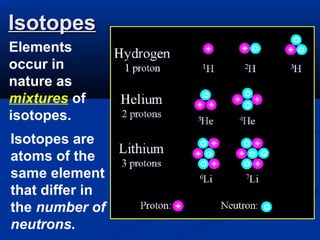
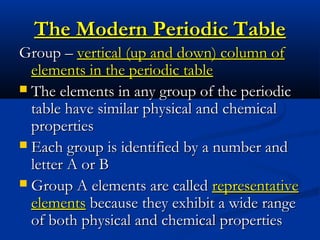
The document discusses the history and development of atomic theory and the periodic table. It describes early atomic models proposed by Democritus and Dalton. Rutherford's gold foil experiment provided evidence that atoms have a small, dense nucleus containing protons and neutrons. Elements are distinguished by their atomic number and isotopes differ in their number of neutrons. The periodic table organizes elements according to recurring trends in their physical and chemical properties, allowing relationships between elements to be identified.