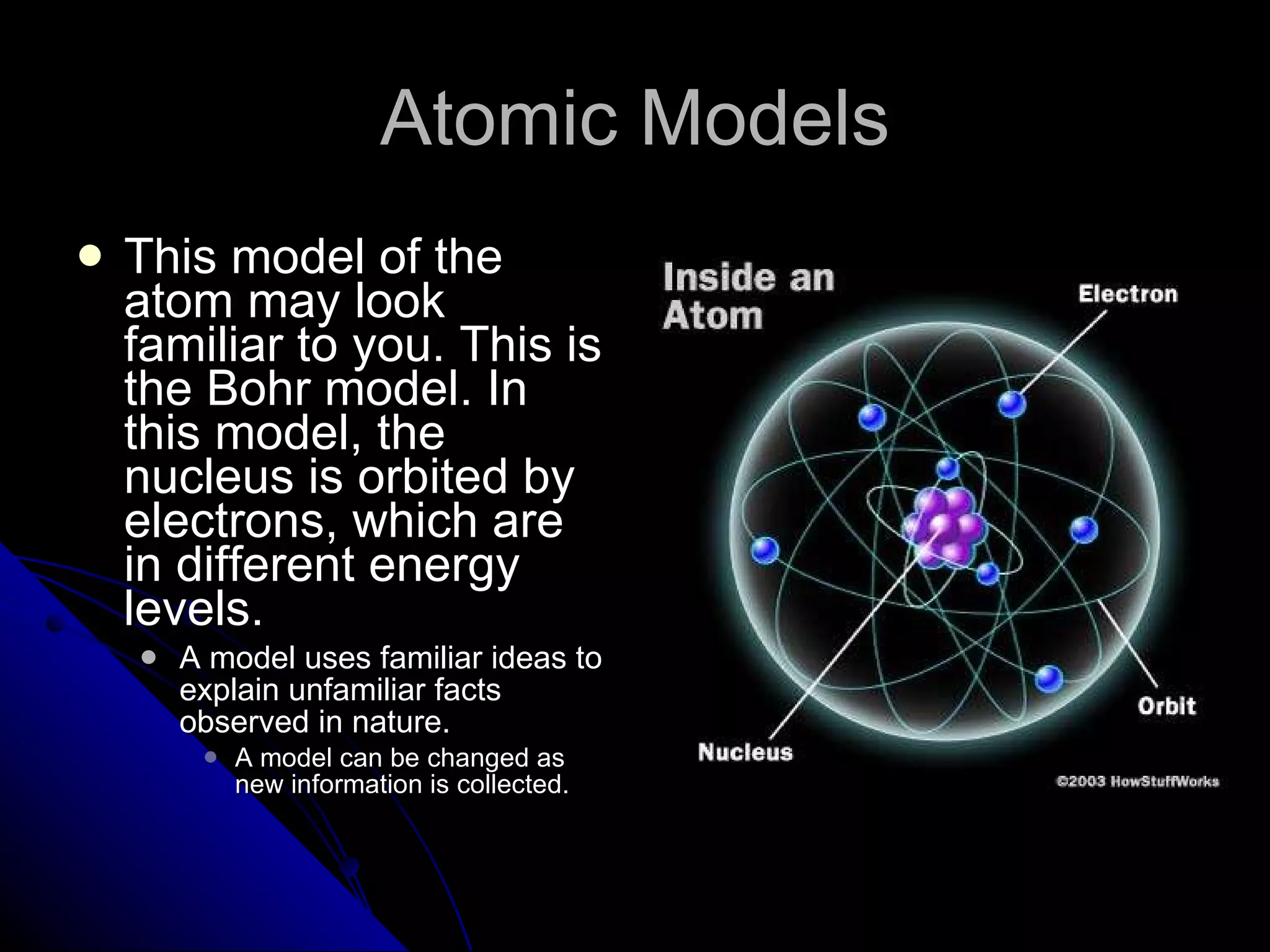
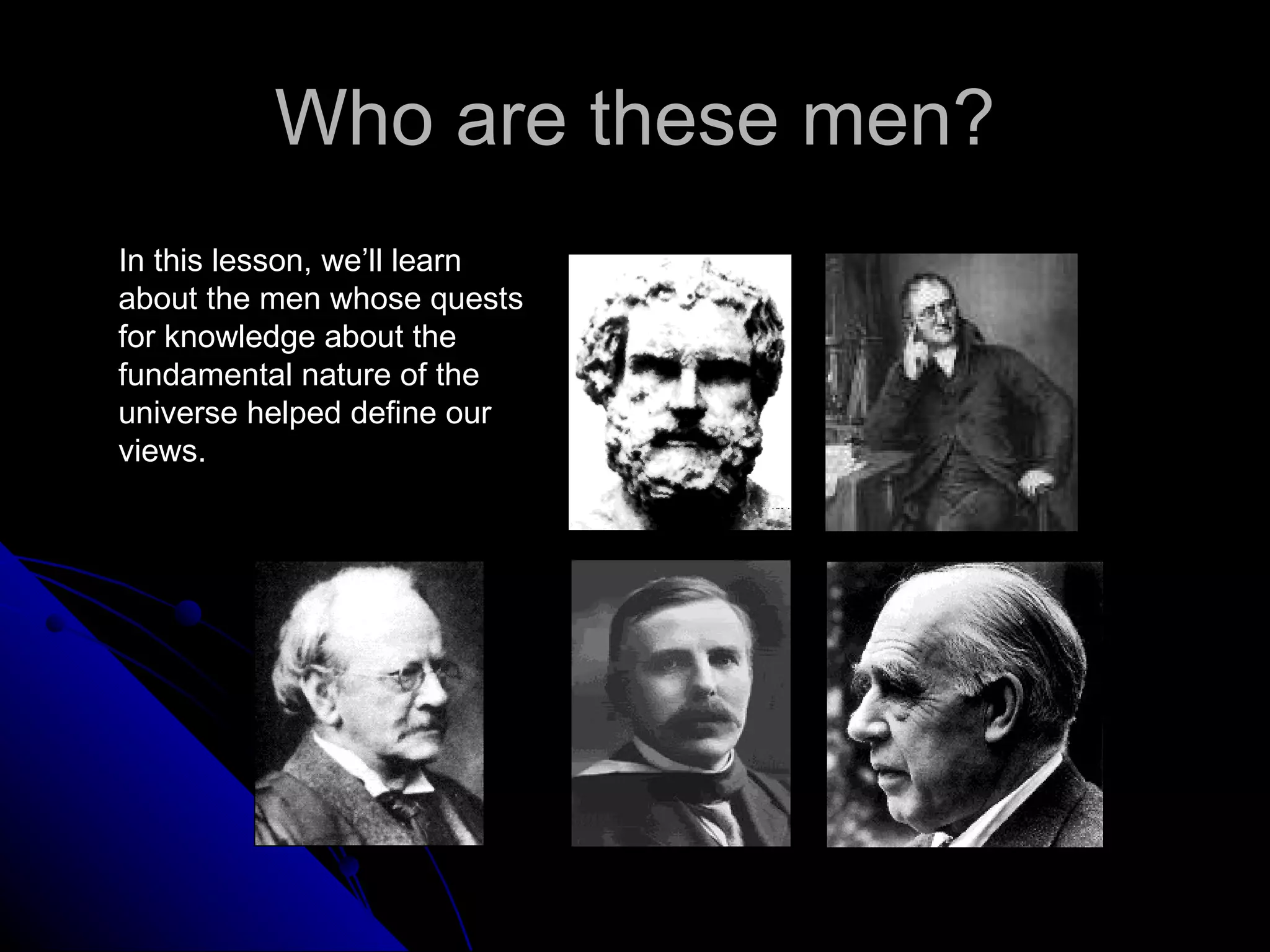
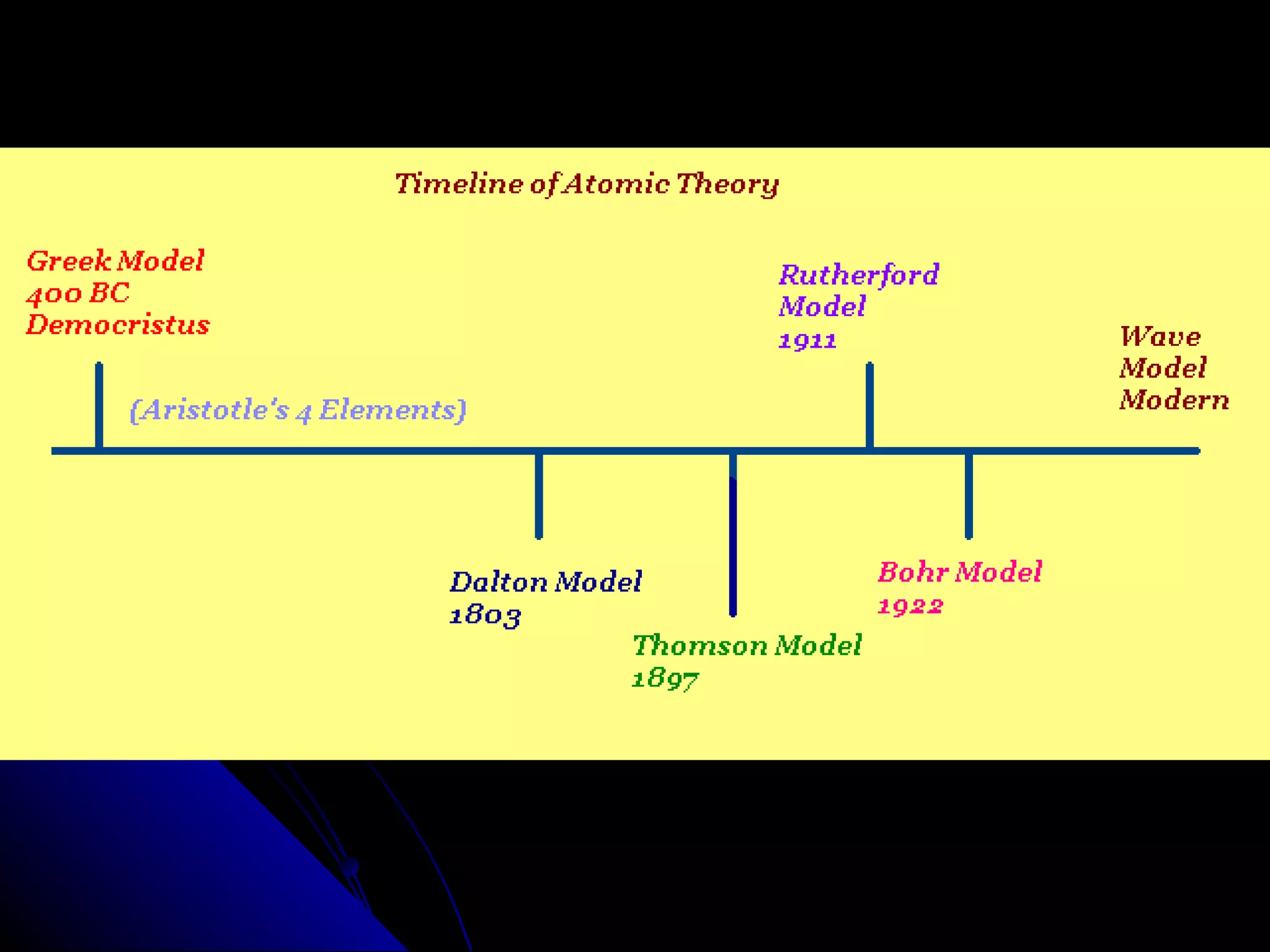
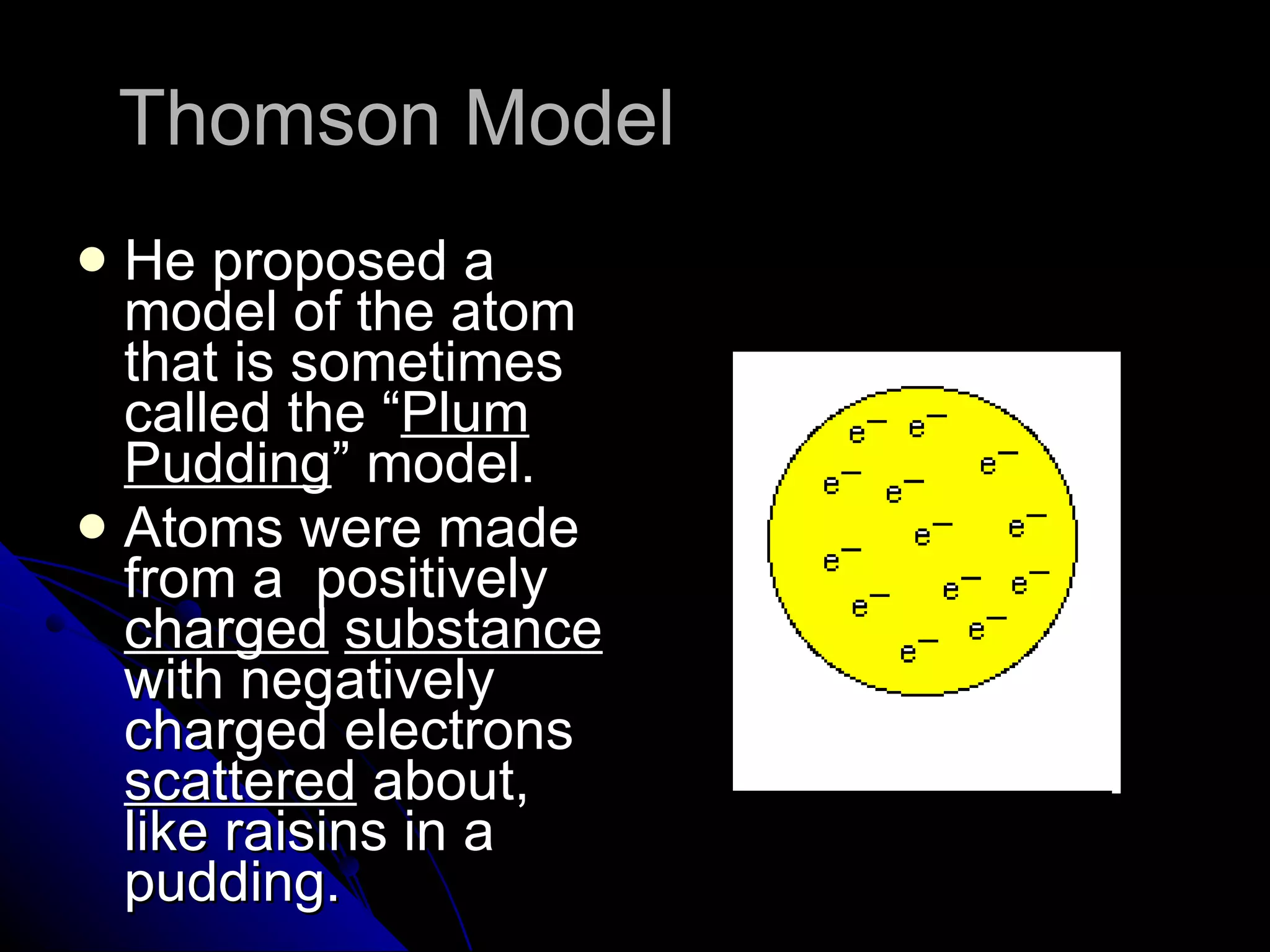
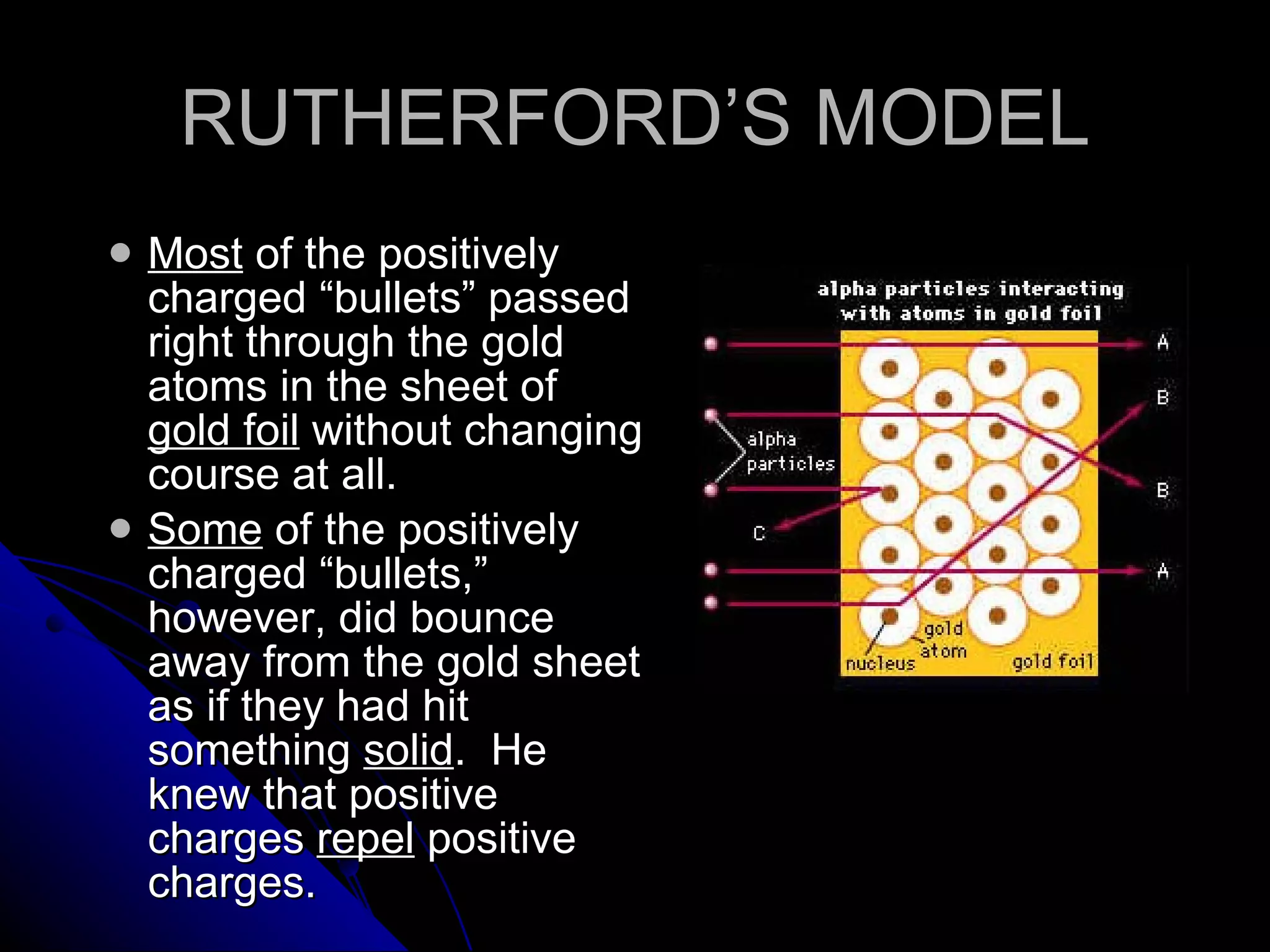
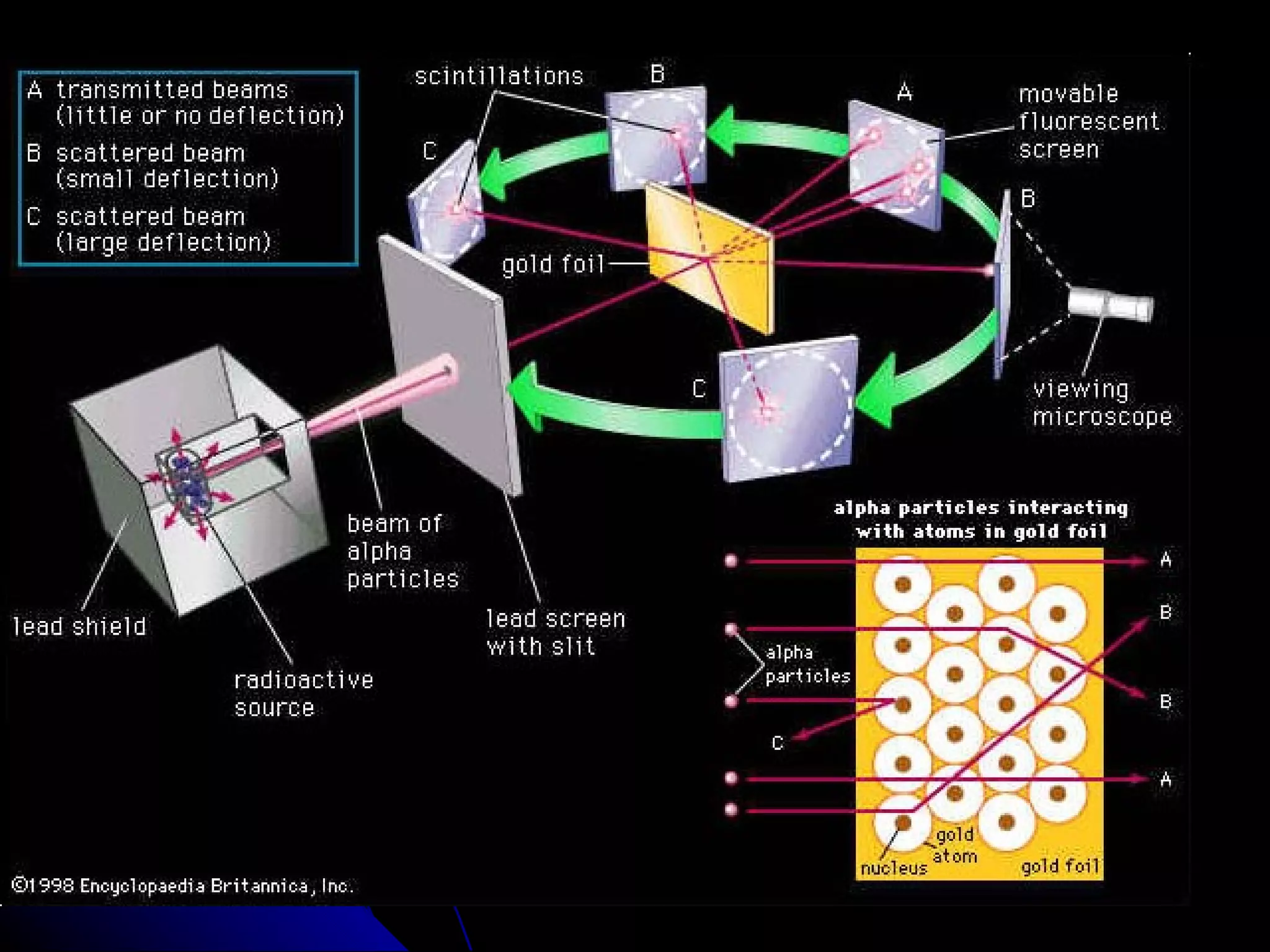
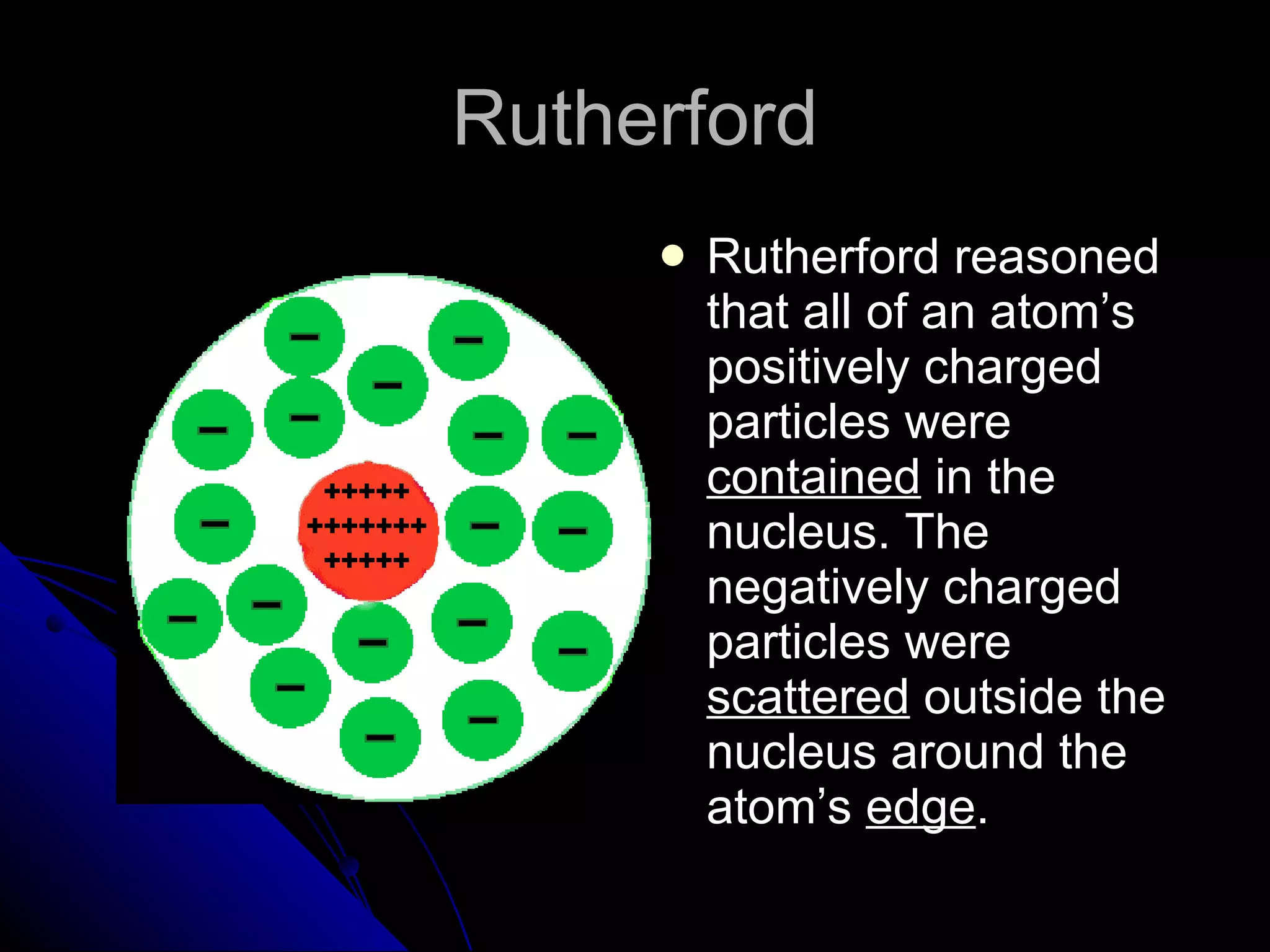
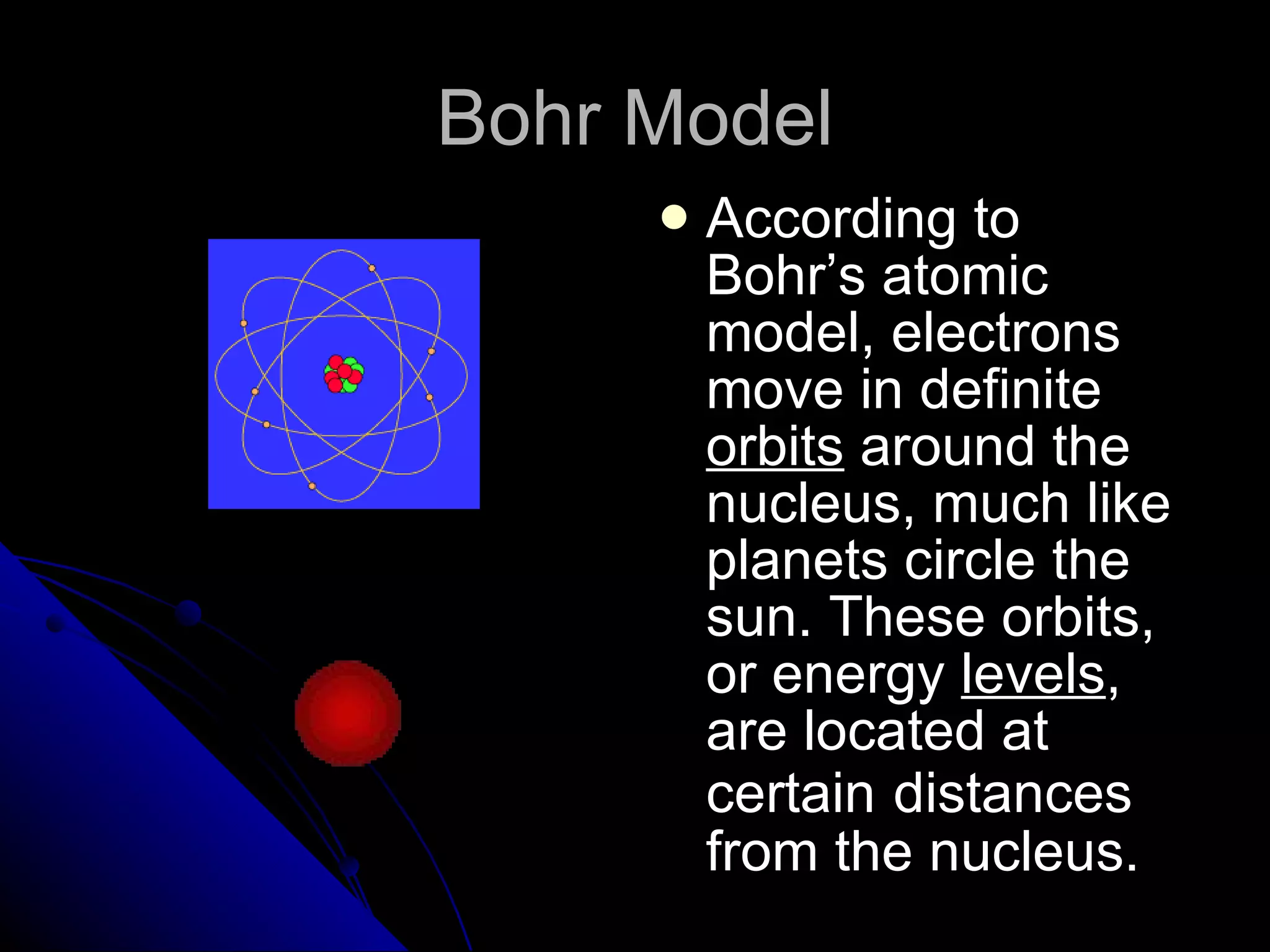
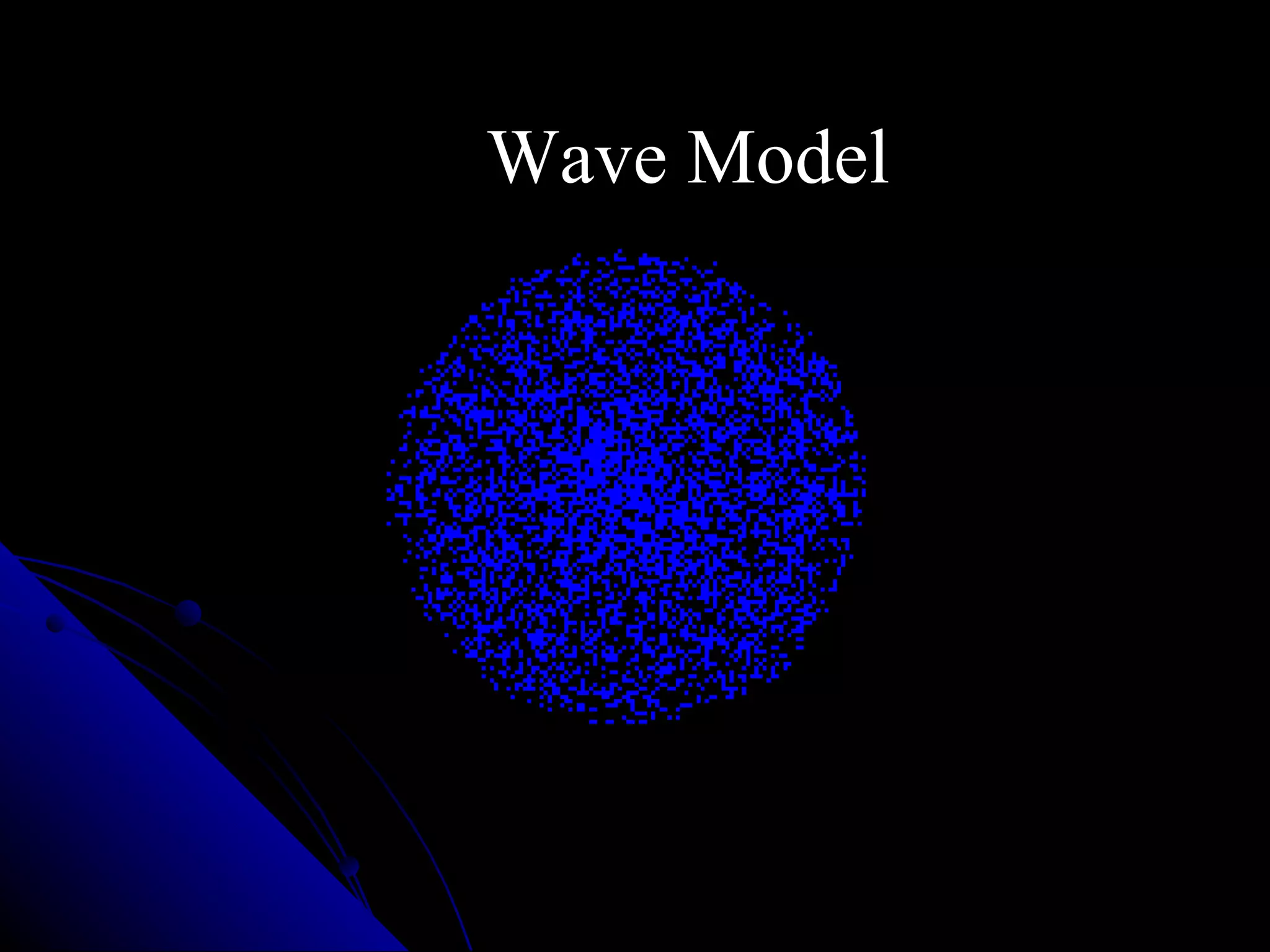
The document traces the development of atomic theory from ancient Greek philosophers to modern physics. Democritus first proposed that matter is made of indivisible "atoms" around 400 BC. In the early 1800s, John Dalton provided experimental evidence supporting atoms and proposed that atoms of different elements have different properties. In the late 1800s and early 1900s, experiments by J.J. Thomson, Ernest Rutherford, and Niels Bohr led to discoveries of the electron and development of the nuclear model of the atom. Today's atomic model is based on quantum mechanics and depicts electrons as existing in electron clouds or energy levels rather than definite orbits.