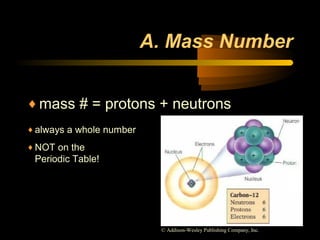
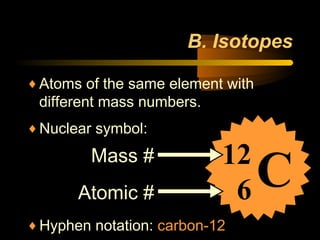
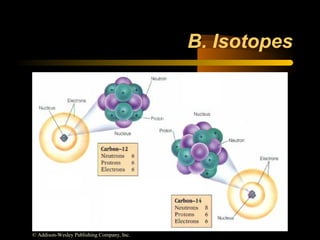
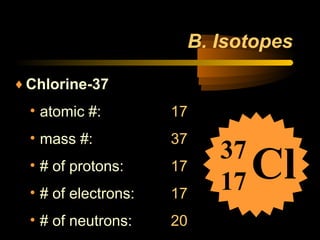
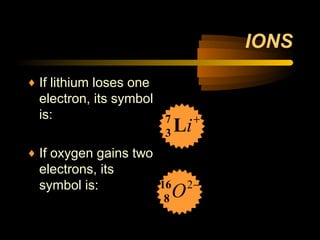
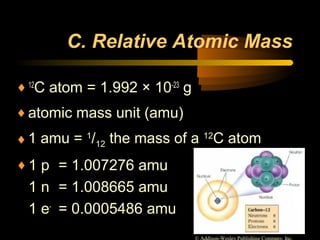
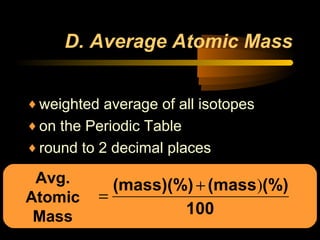
This document discusses atomic structure and masses of atoms. It explains that the mass number of an atom equals the number of protons plus neutrons. Isotopes are atoms of the same element that have different mass numbers. The relative atomic mass of an atom is calculated relative to 1/12 the mass of a carbon-12 atom. To calculate the average atomic mass of an element, the percentage and atomic mass of each isotope is determined and a weighted average is calculated.