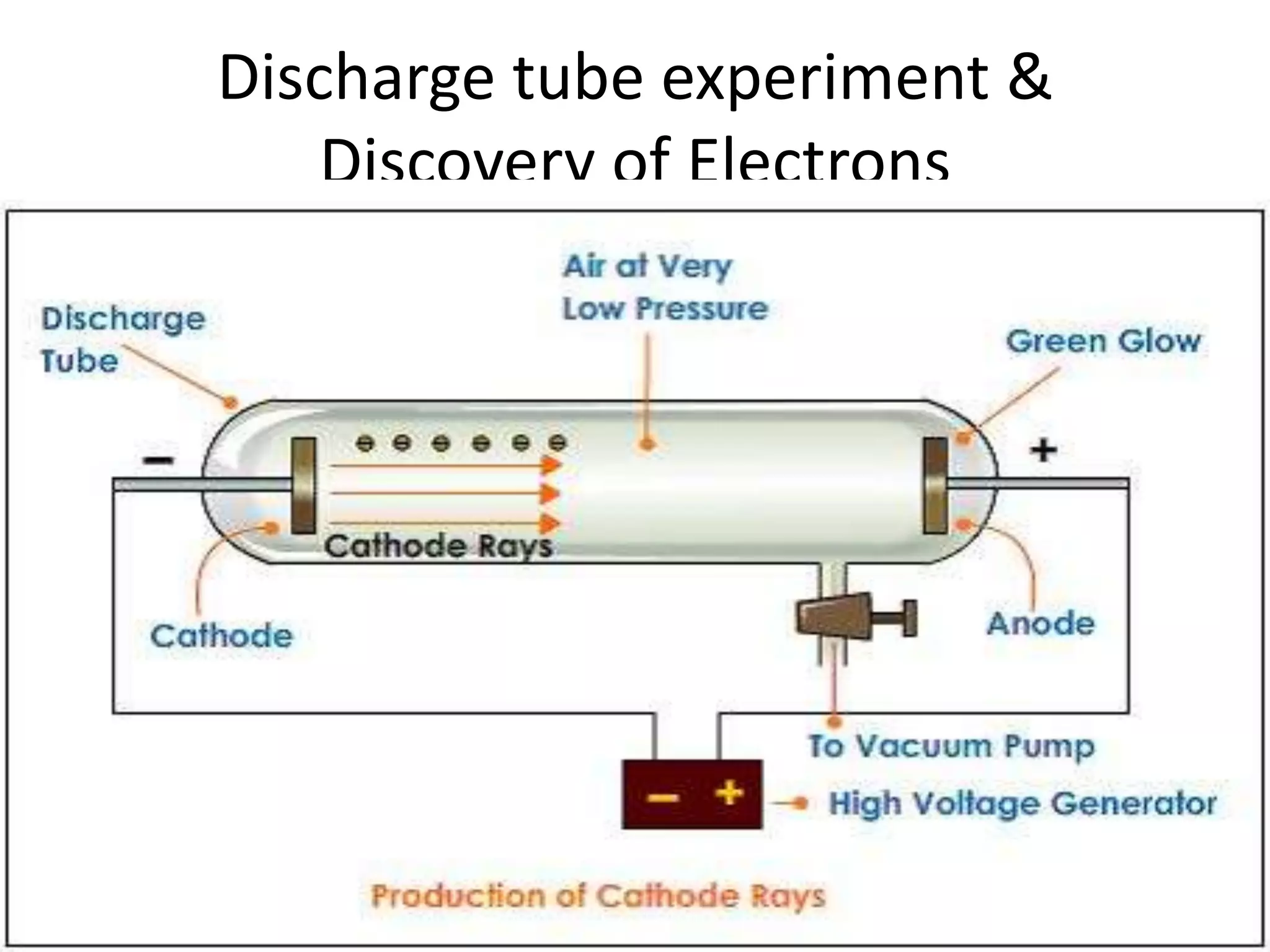
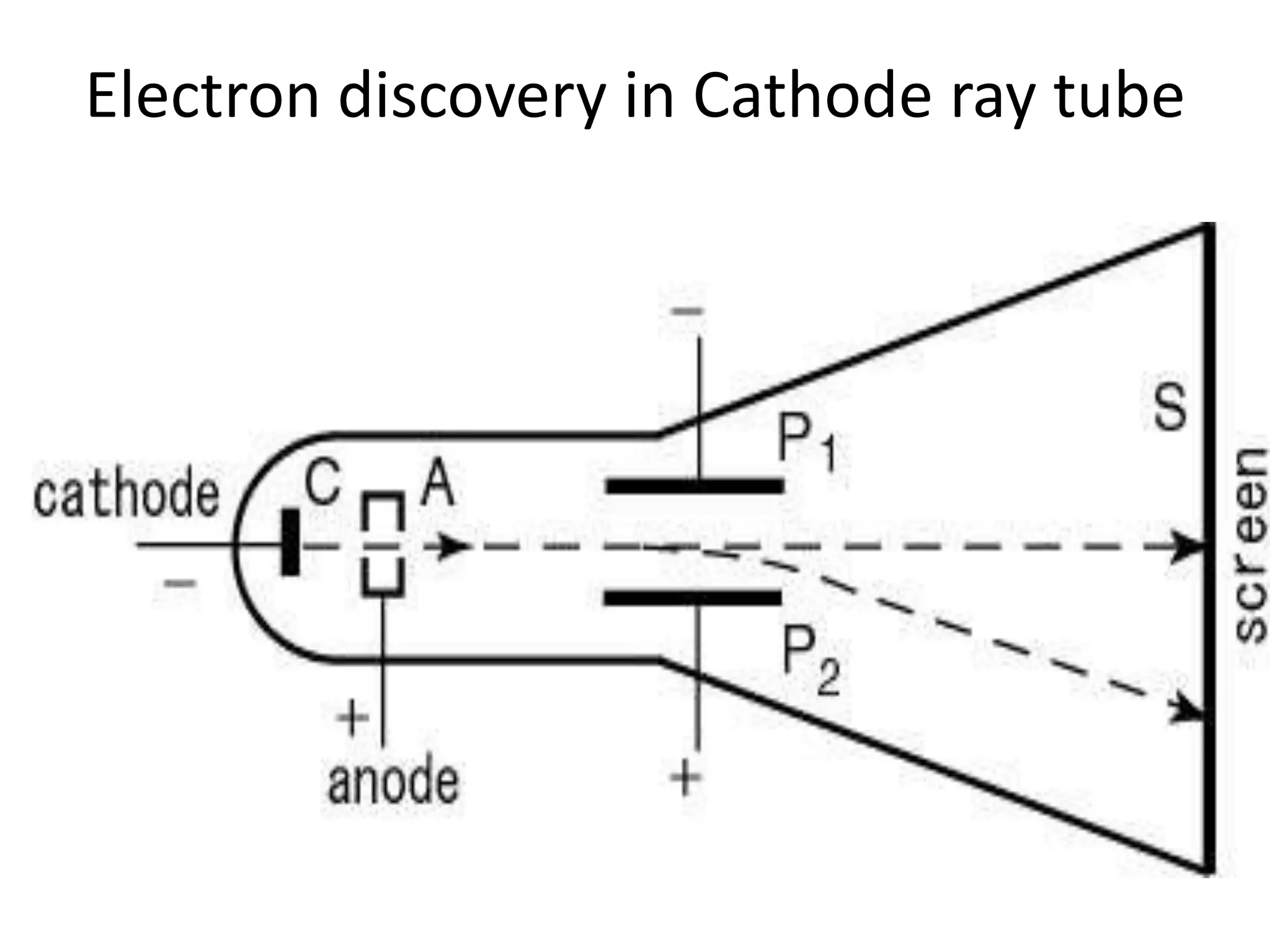
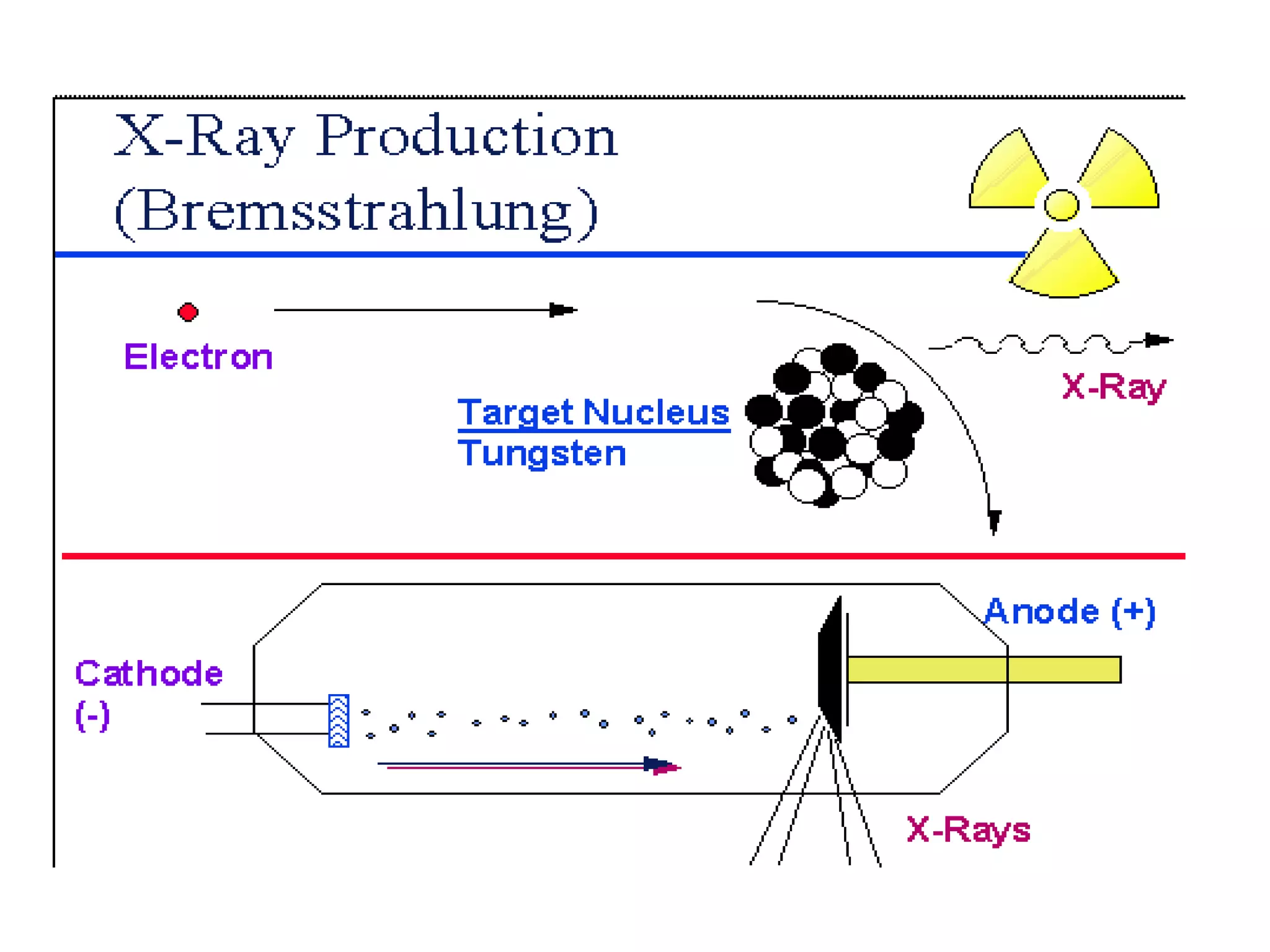
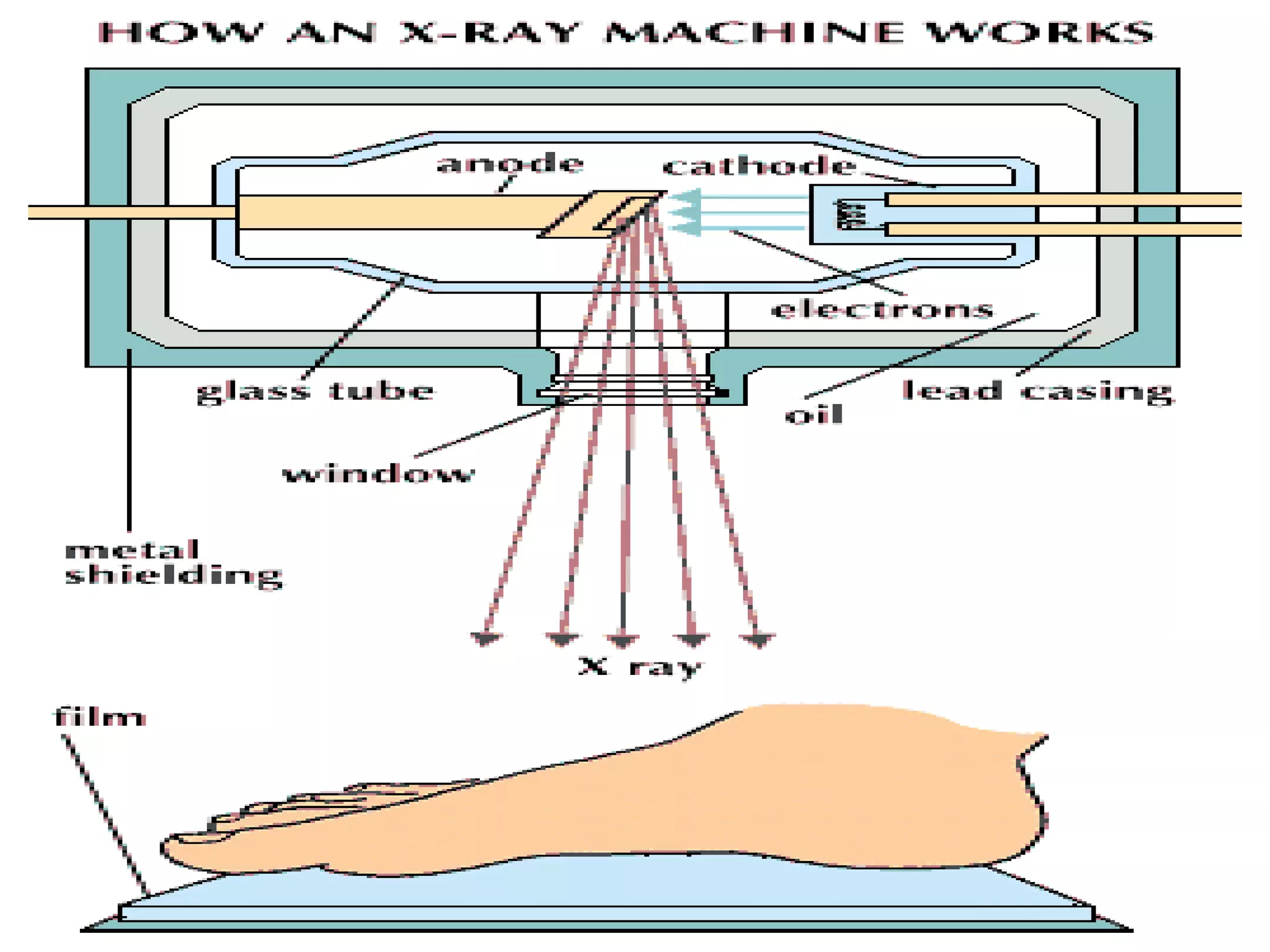
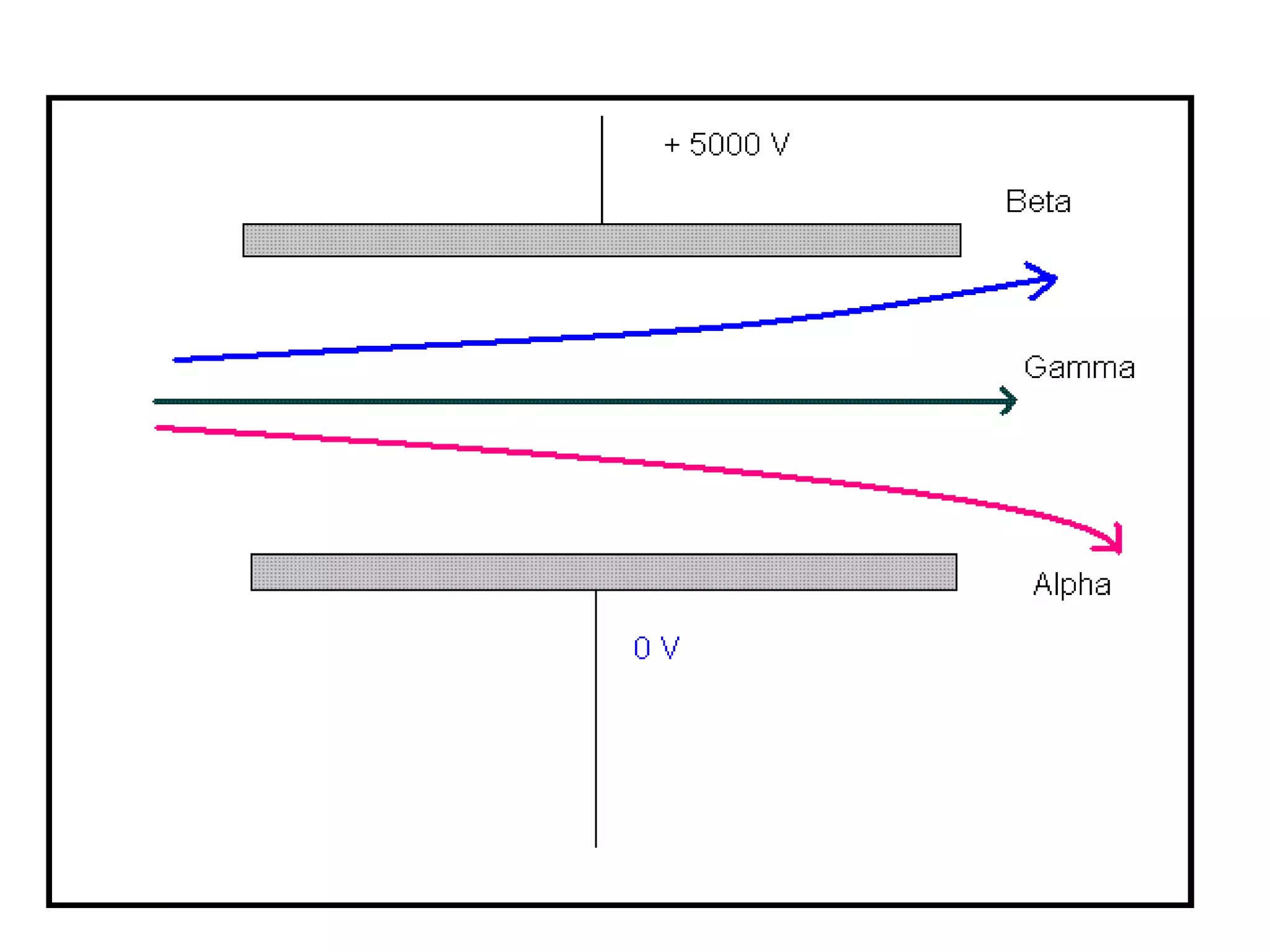
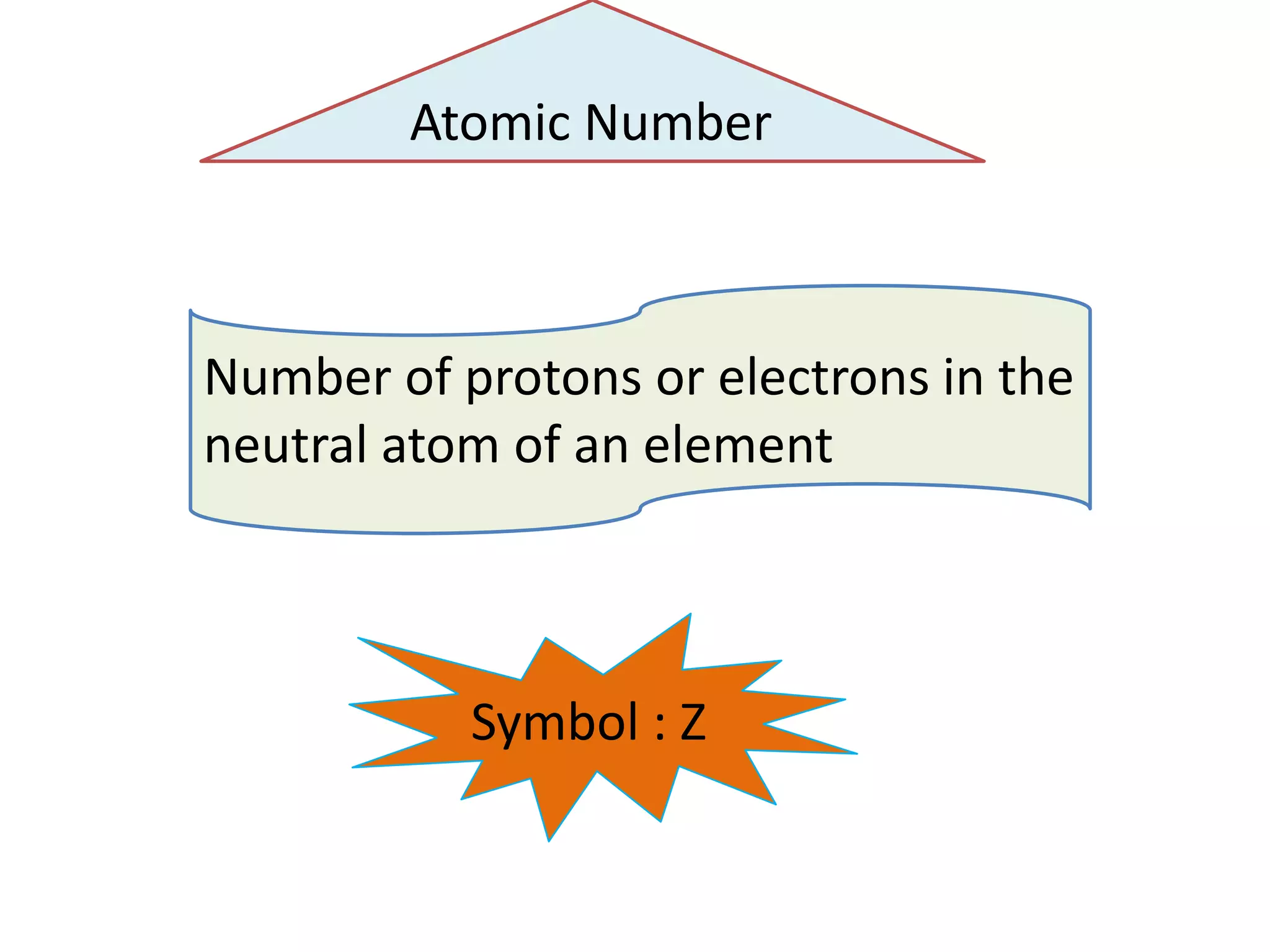
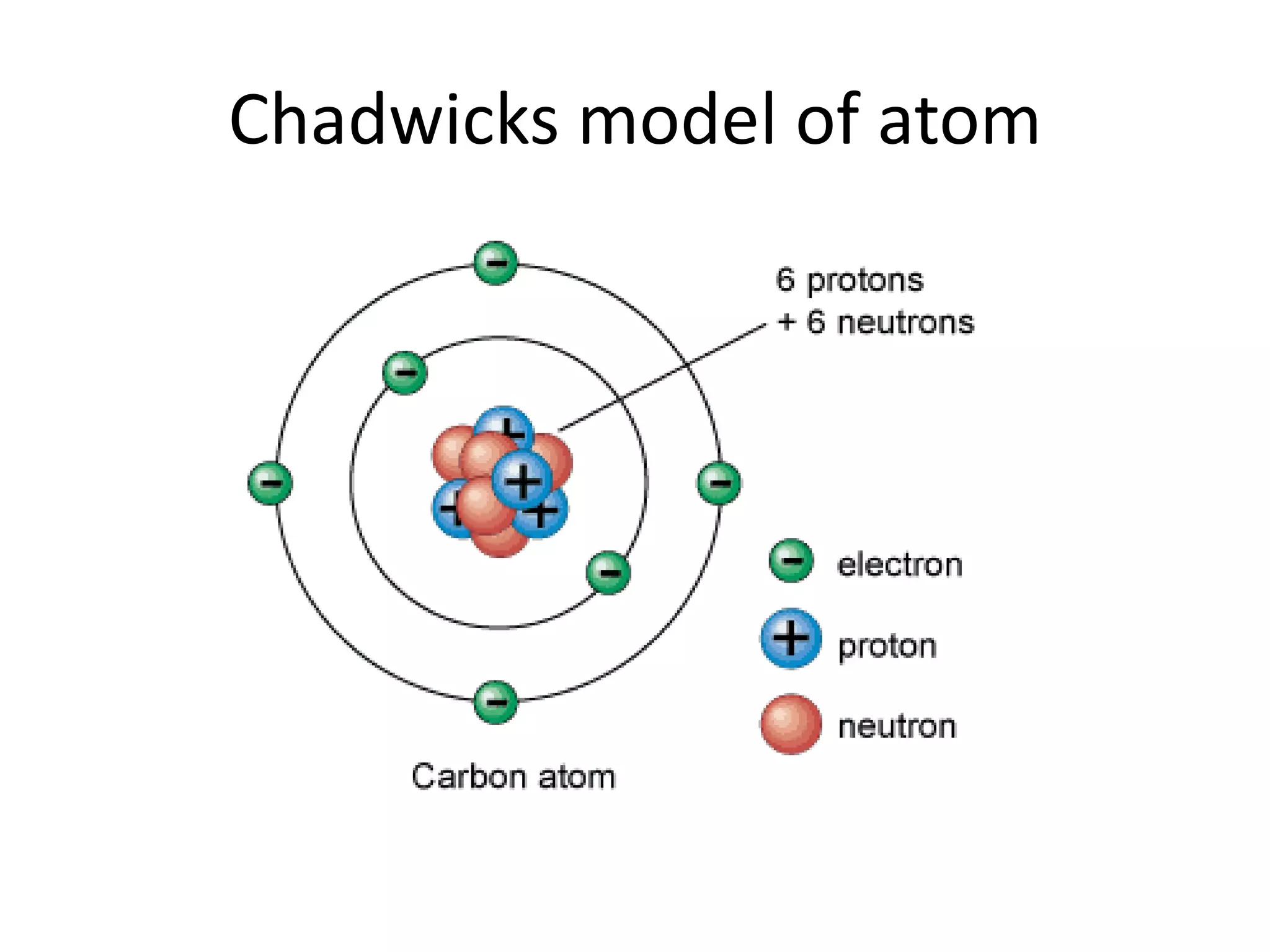
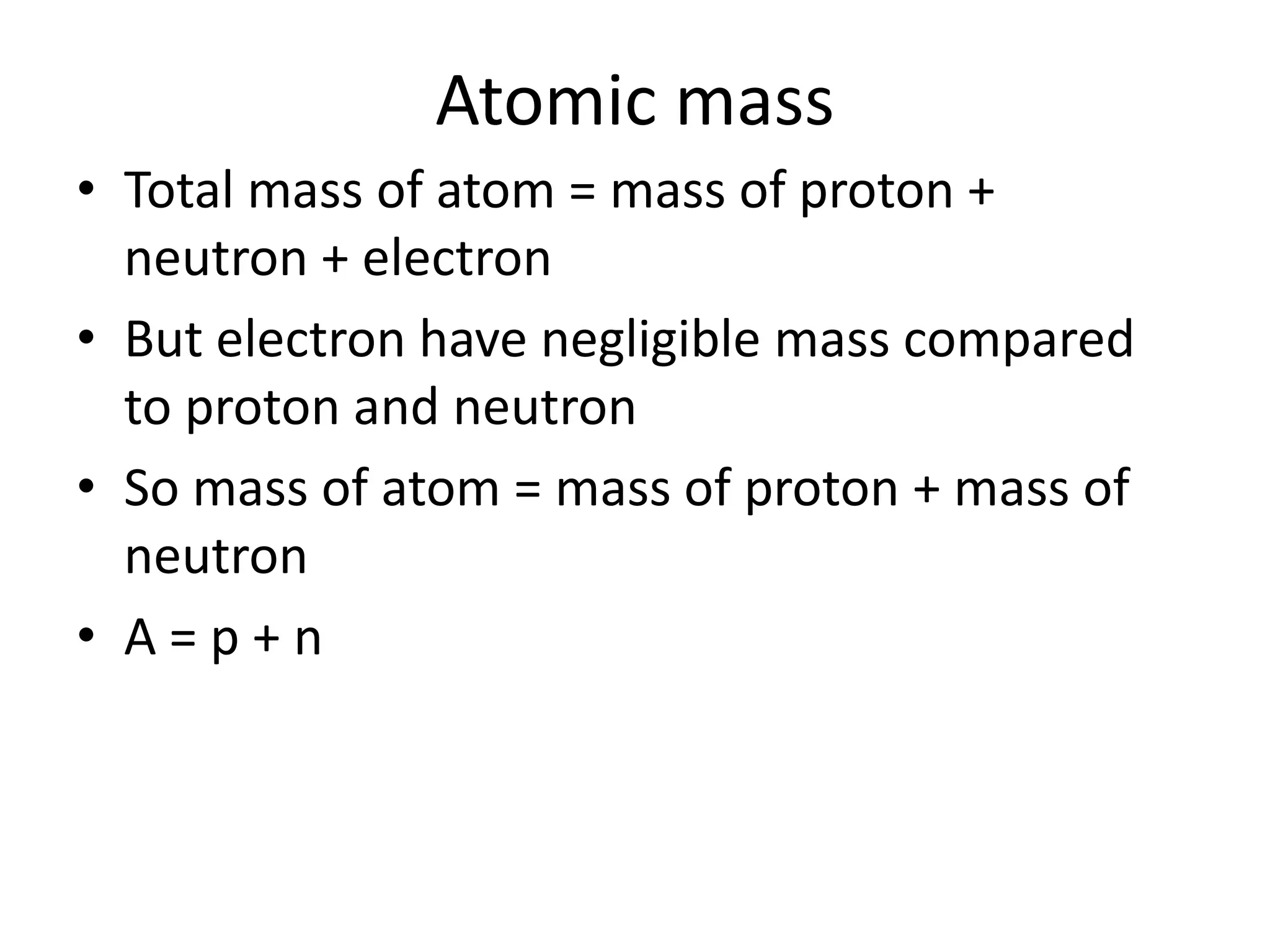
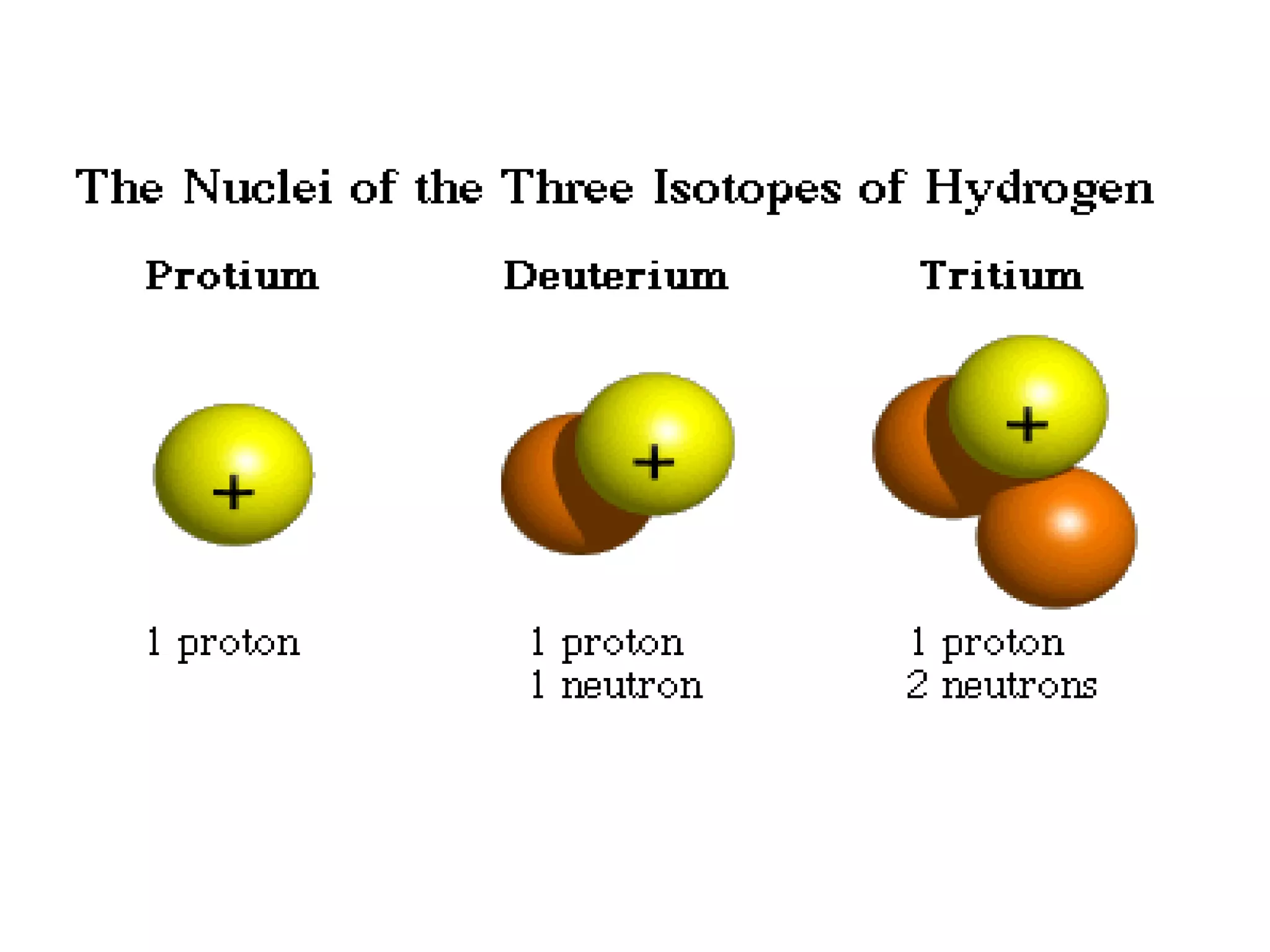
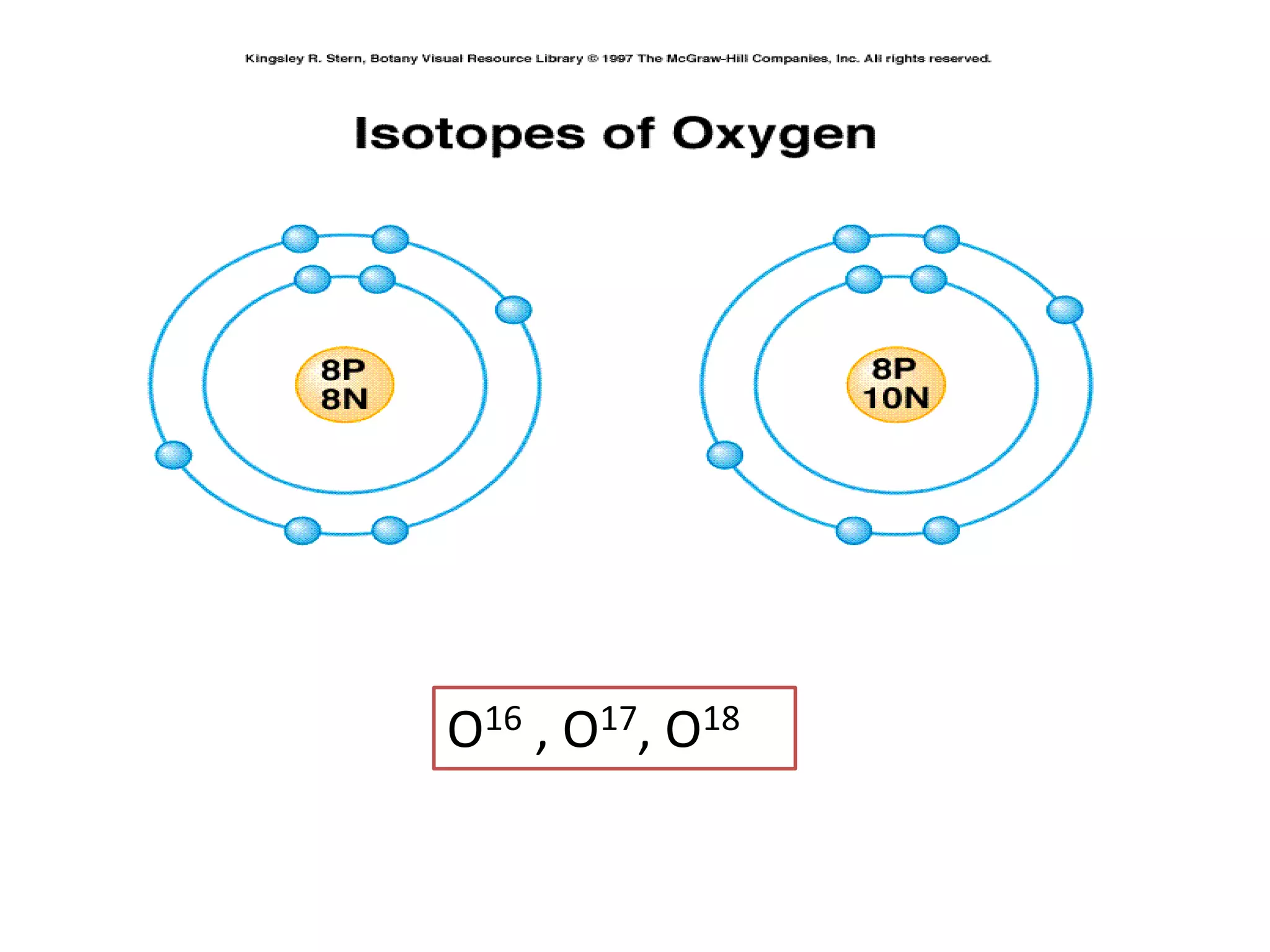
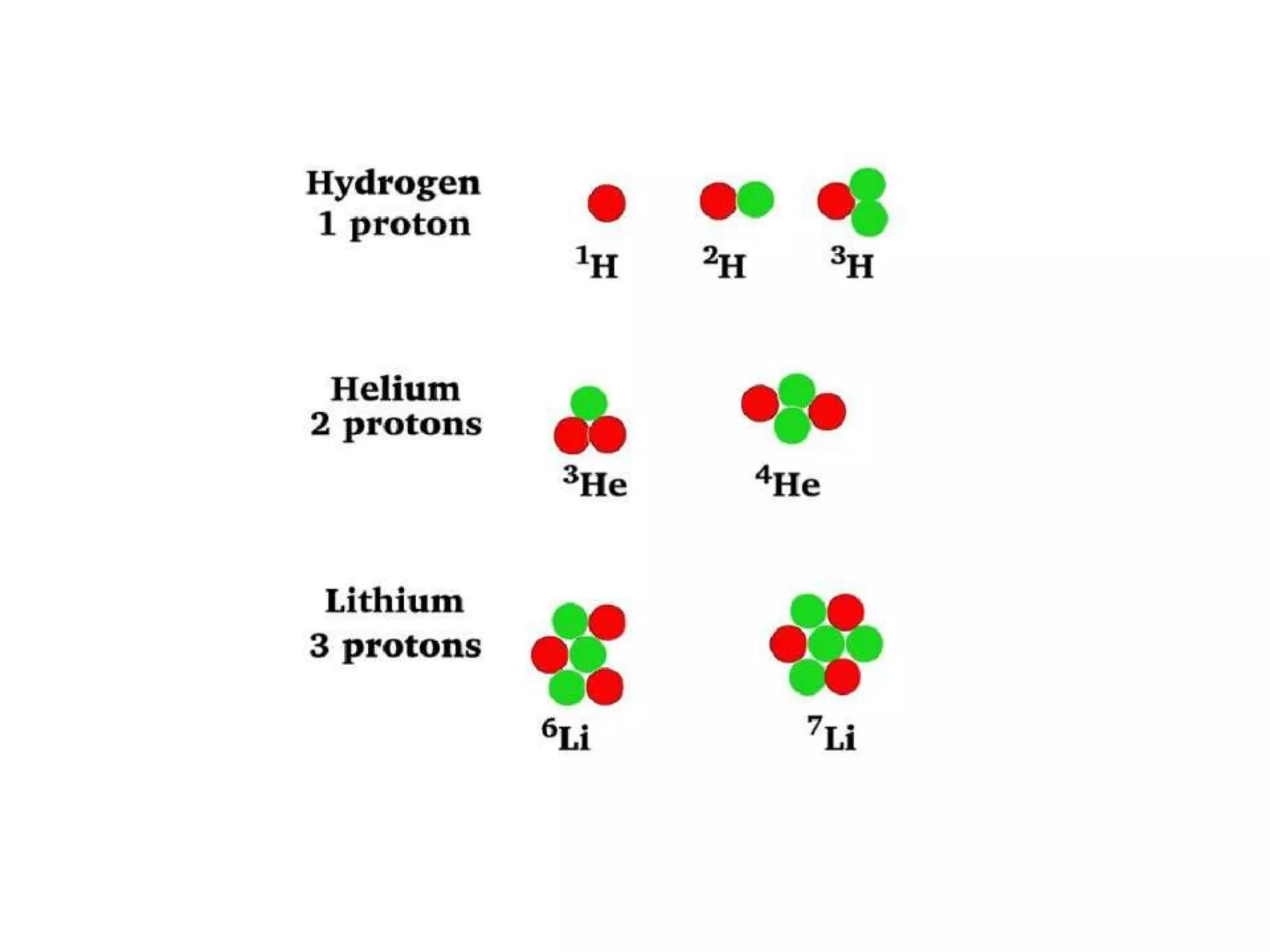
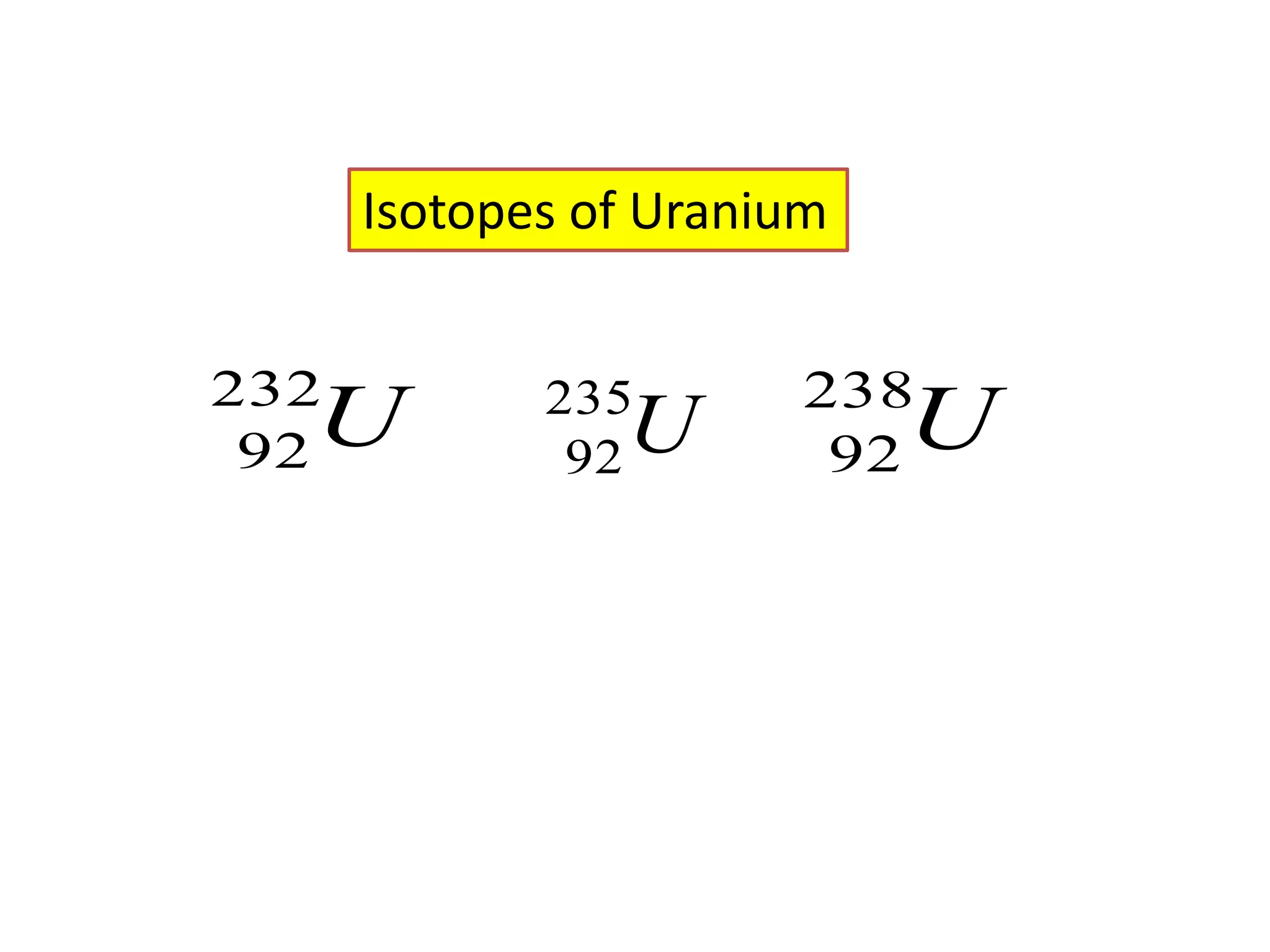
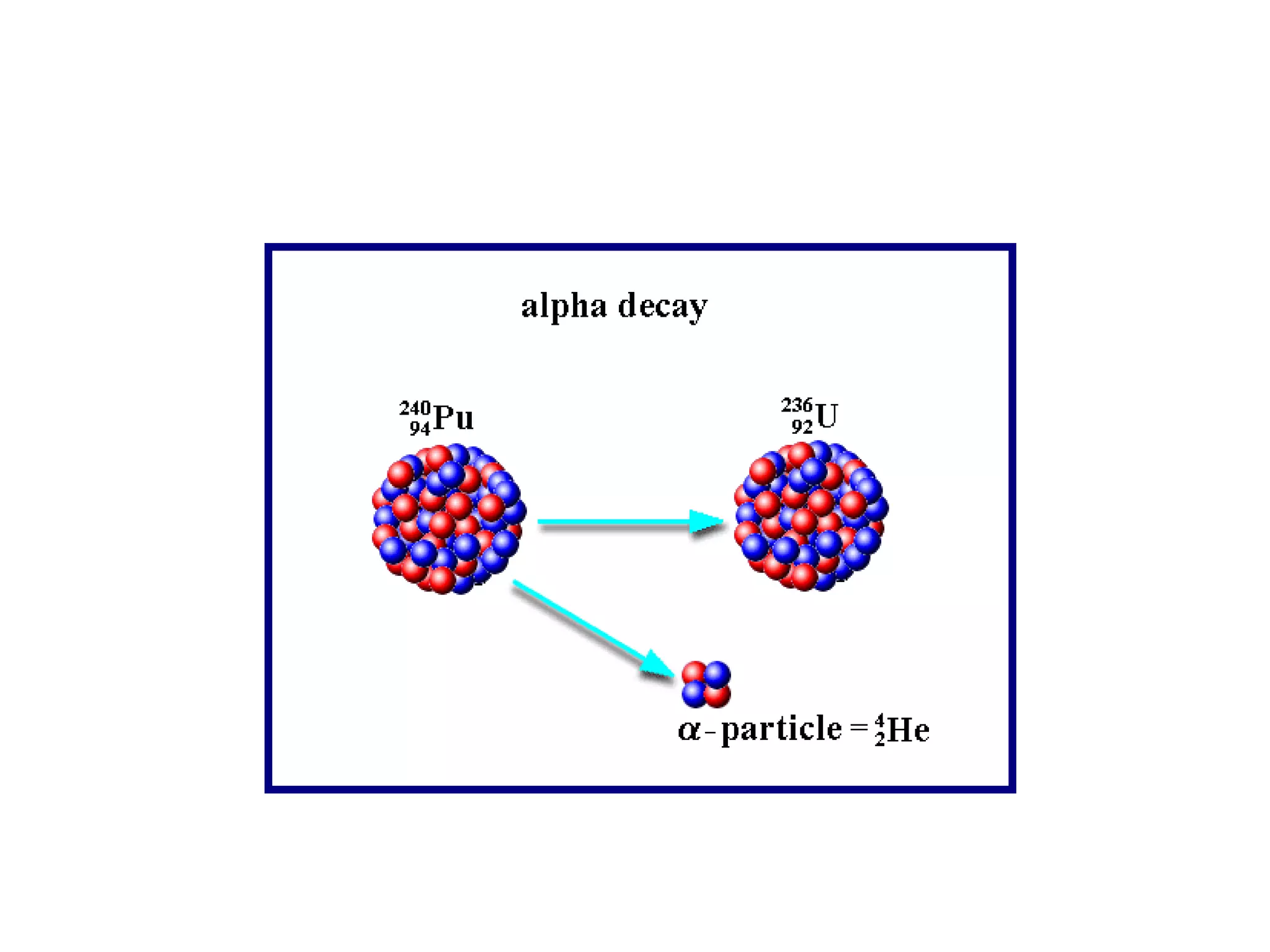
1) The document discusses the evolution of atomic theory from Thomson's model to Bohr's model. It describes key experiments such as cathode rays, X-rays, alpha and beta rays that contributed to scientific understanding of the atom.
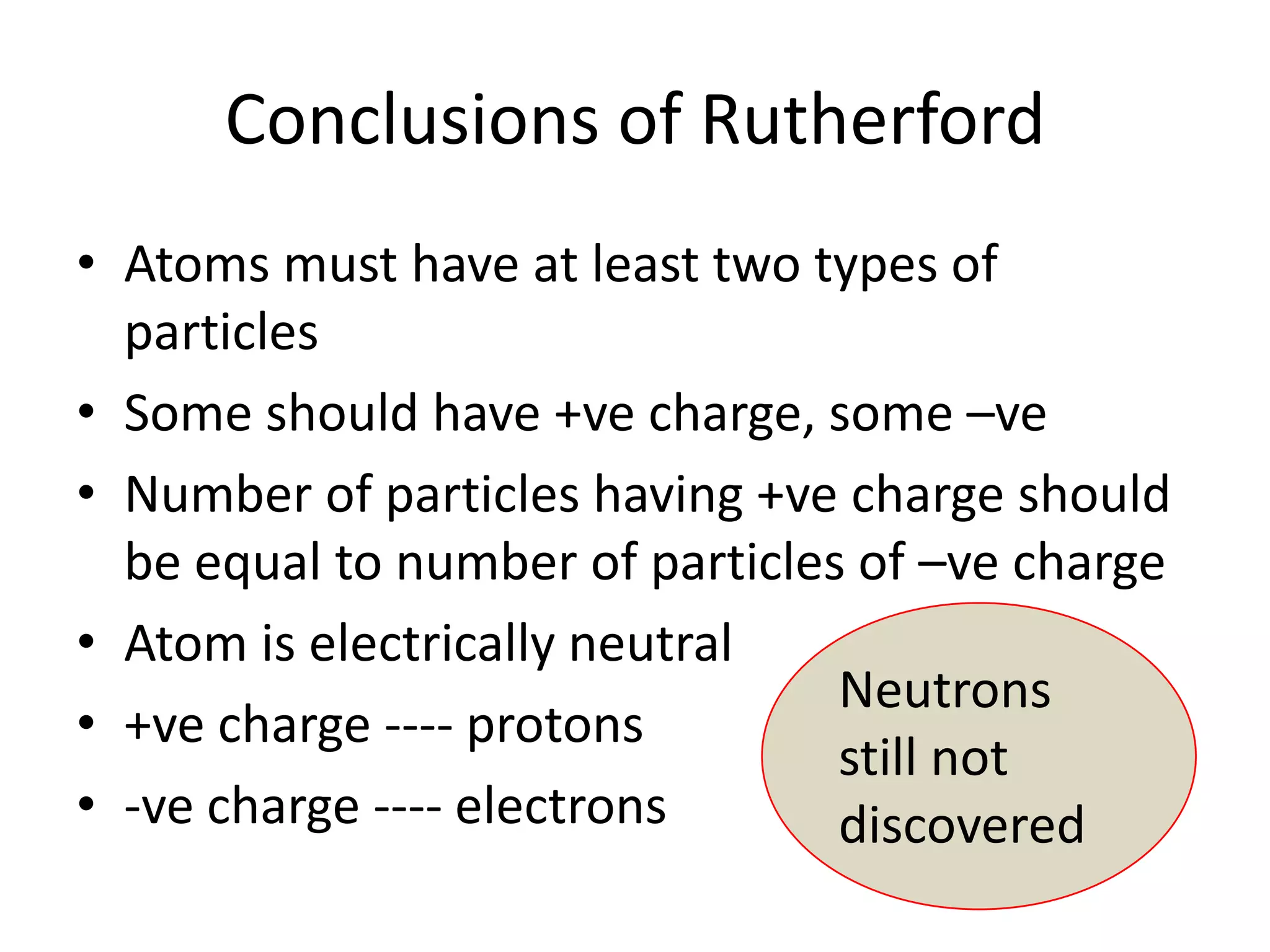
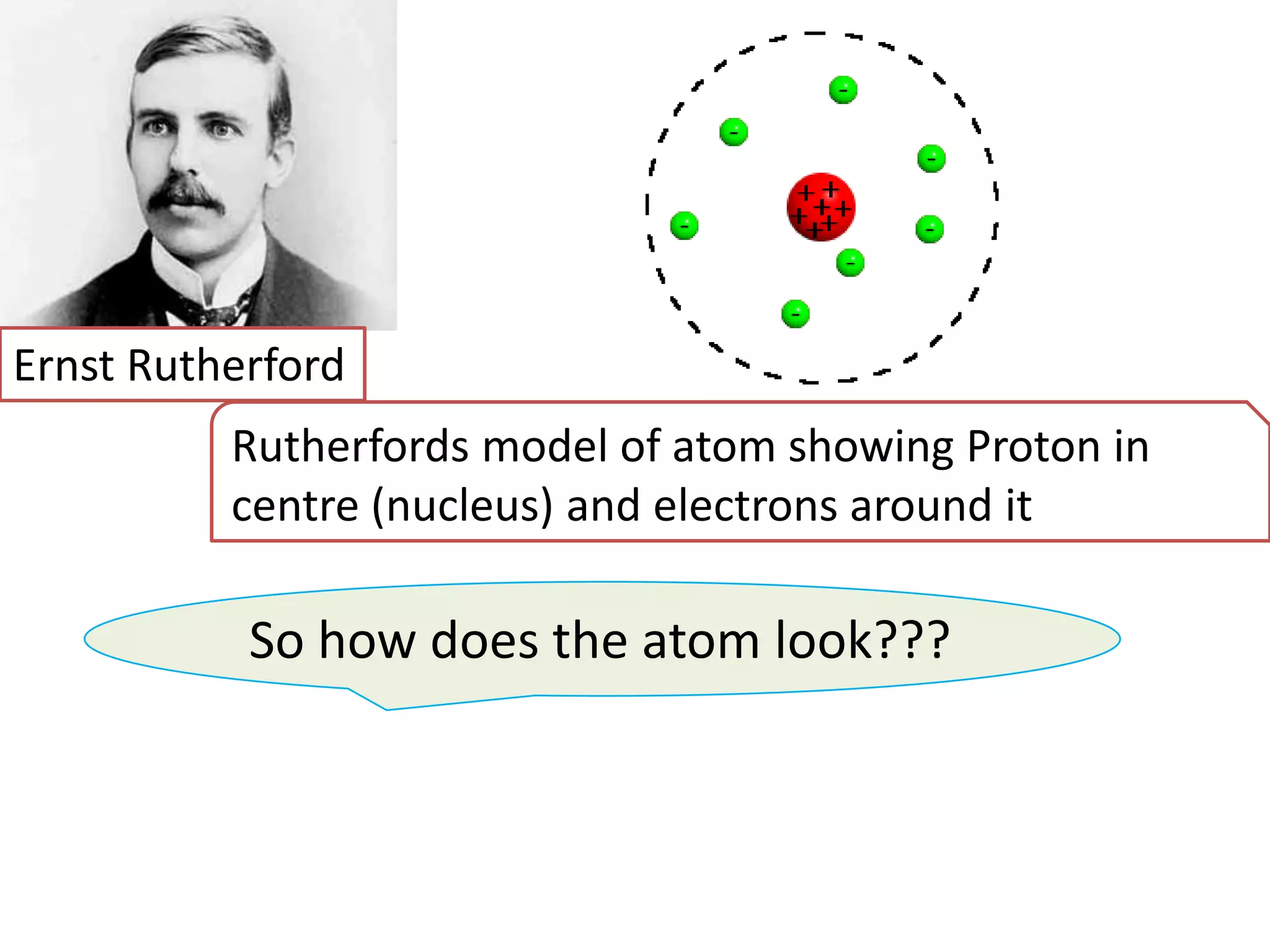
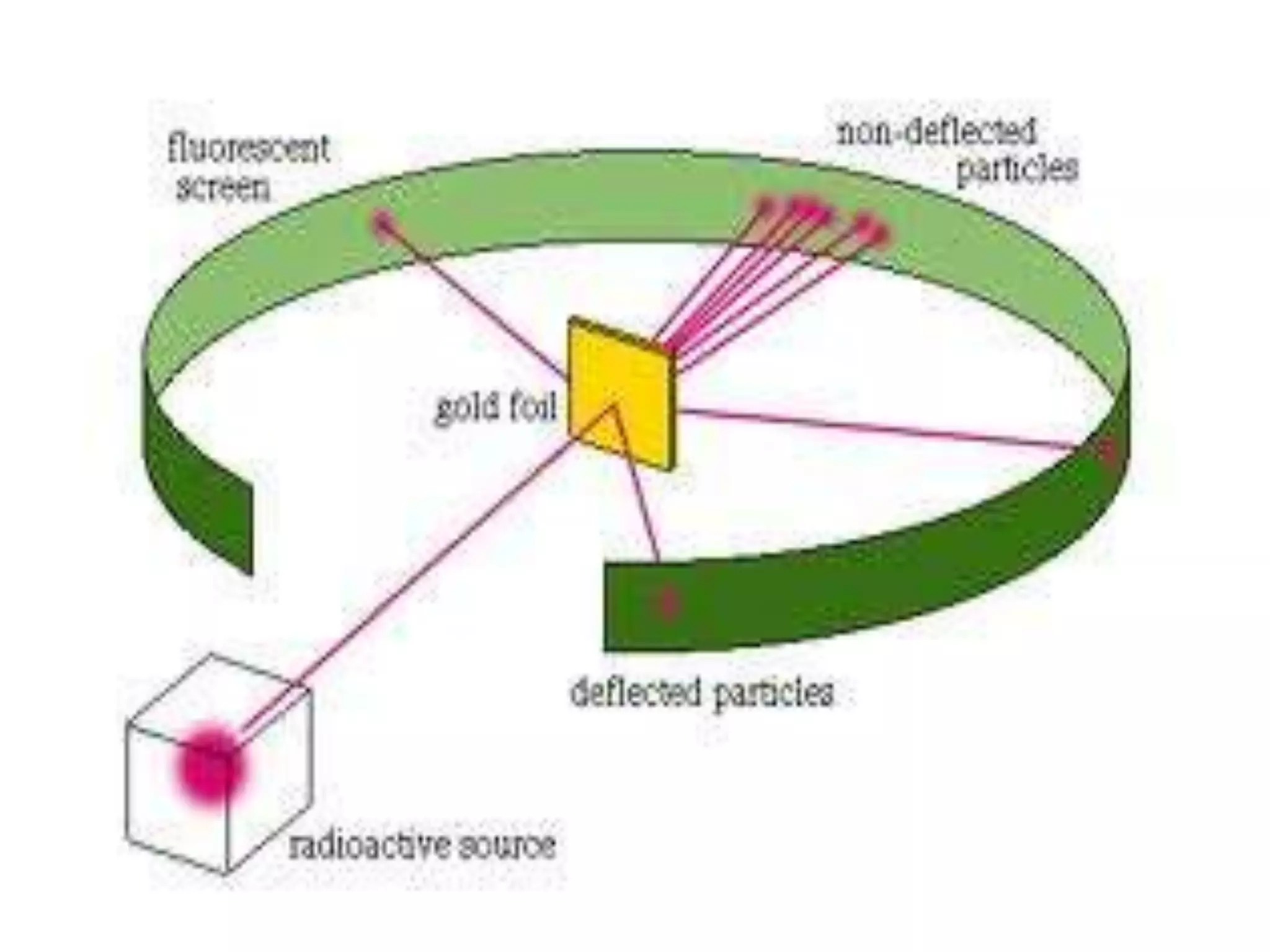
2) Rutherford's gold foil experiment disproved Thomson's uniform sphere model of the atom and established that the atom has a small, dense nucleus at its center with electrons in orbit around it.
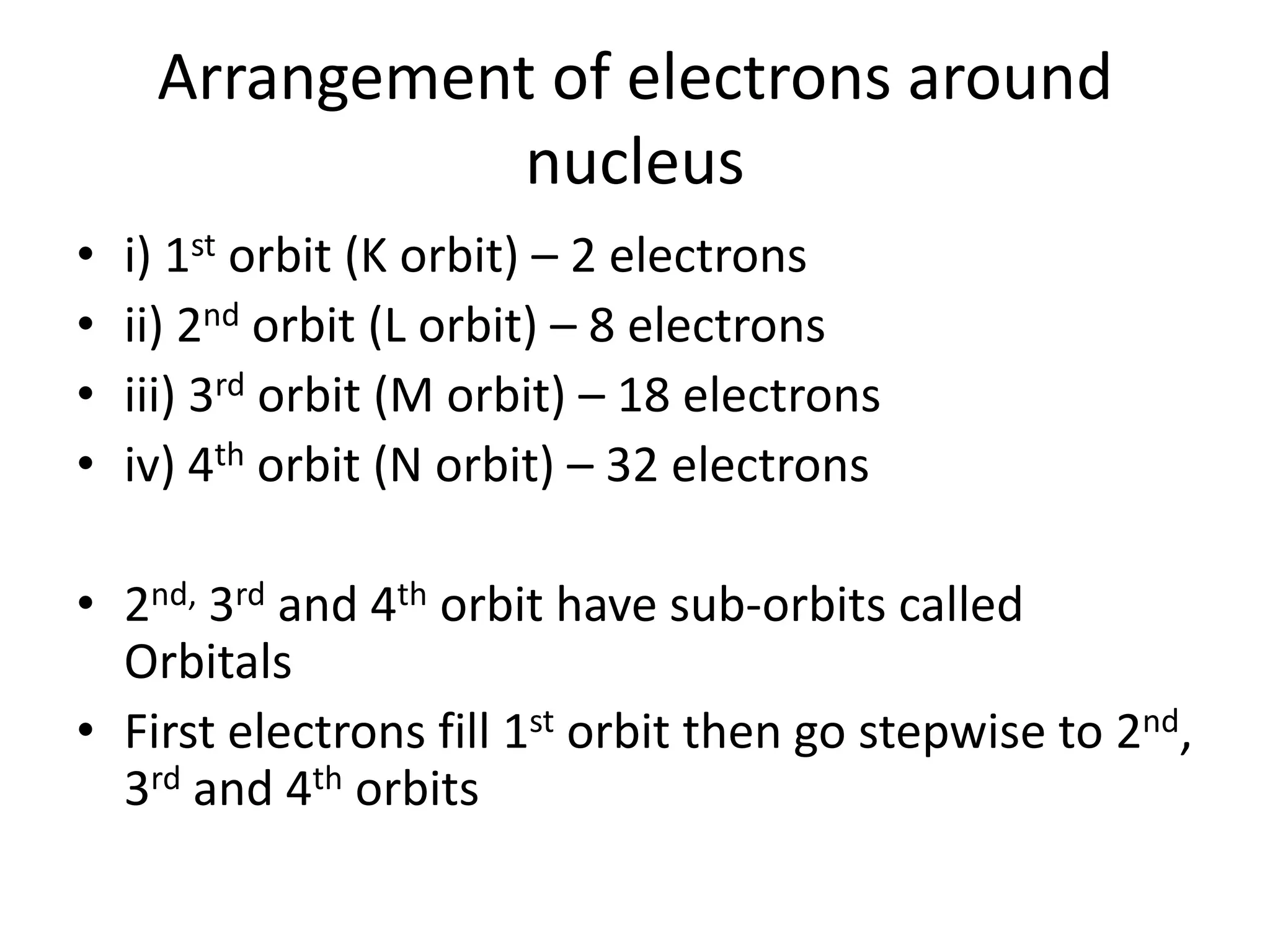
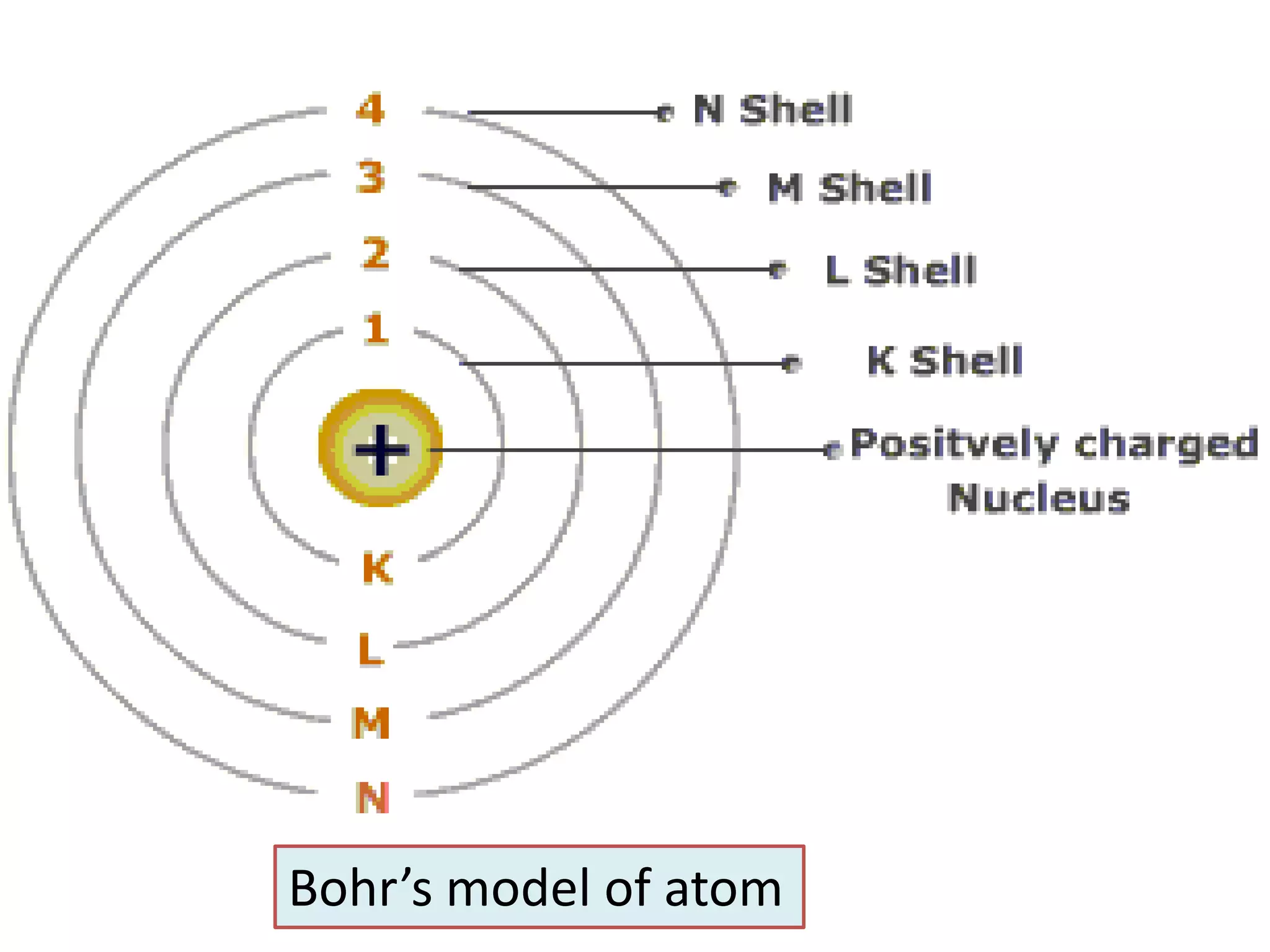
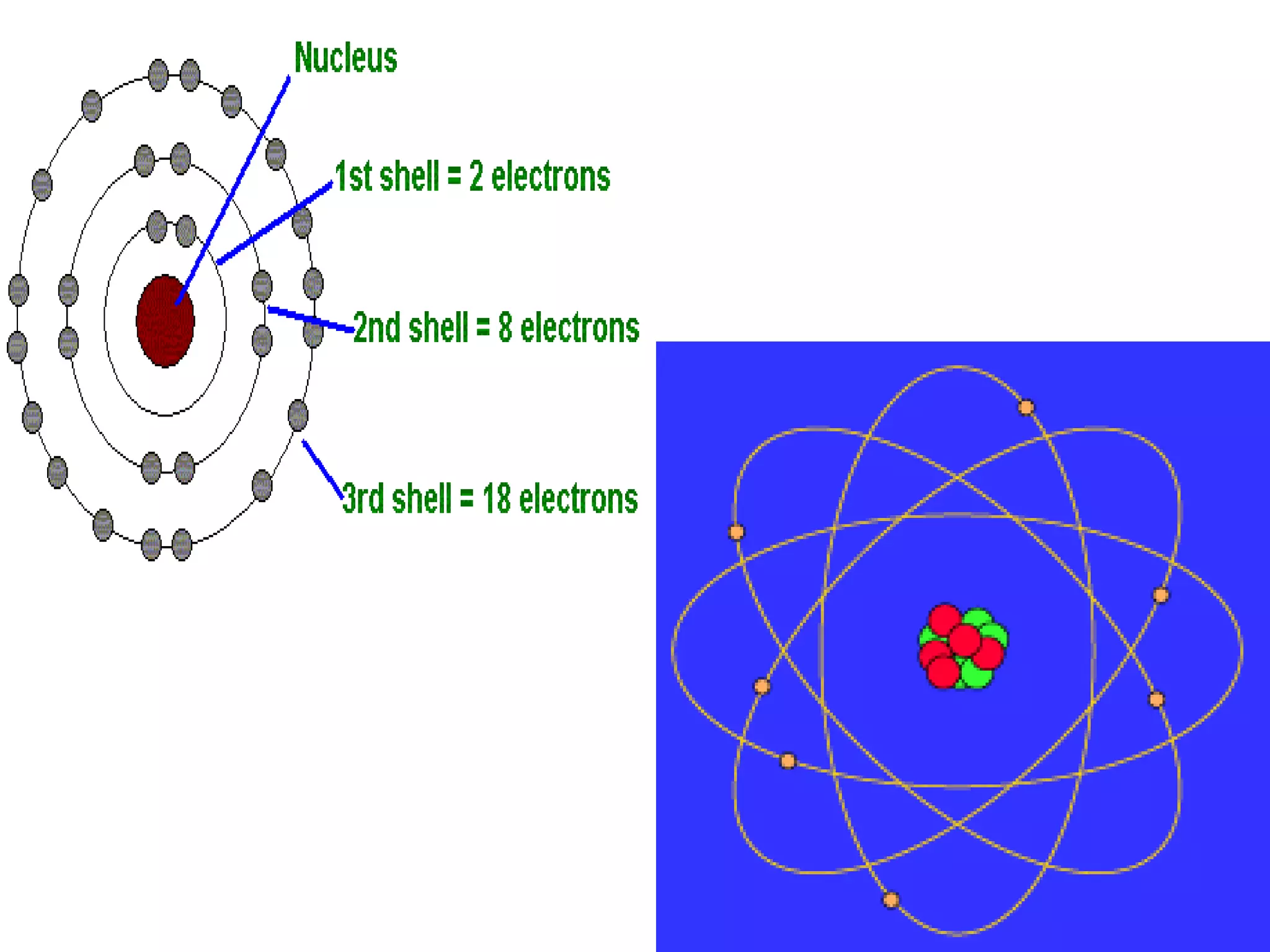
3) Later models such as Bohr's incorporated the concept of electron shells and energy levels to explain the arrangement of electrons in an atom.