This document summarizes key concepts from Chapter 4 on atomic structure:
1) It describes early atomic theories from Democritus and Dalton, including Dalton's postulates that atoms are indivisible and atoms of different elements have different properties.
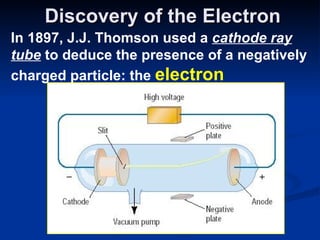
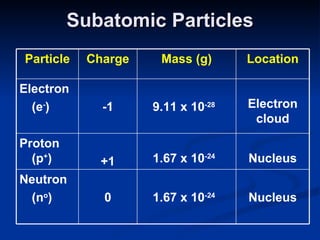
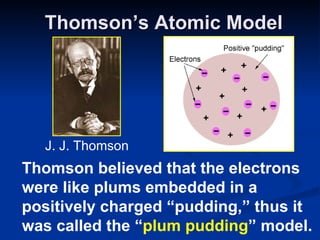
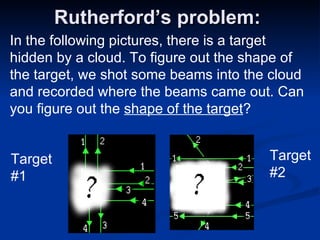
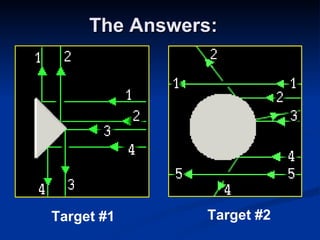
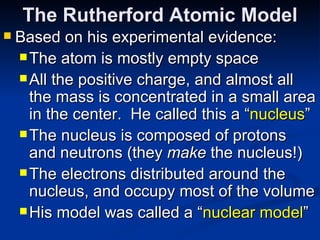
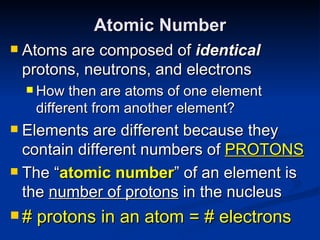
2) Modern research has shown atoms are composed of subatomic particles like electrons, protons, and neutrons. Experiments by Thomson, Millikan, Rutherford and others led to discoveries about these particles and the nuclear model of the atom.
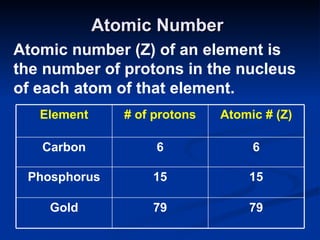
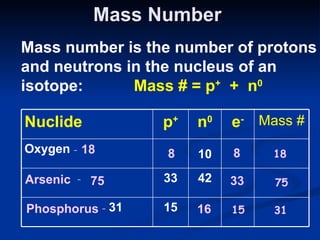
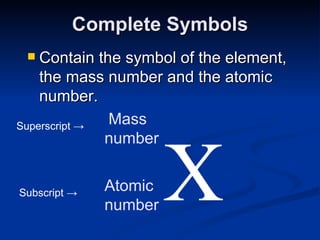
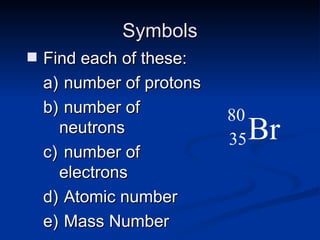
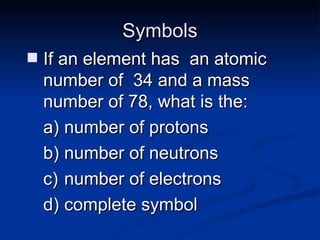
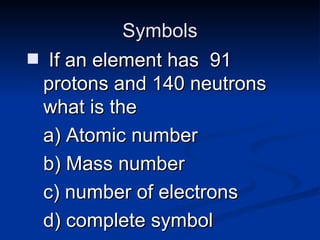
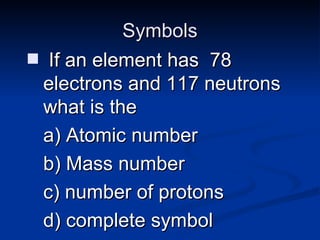
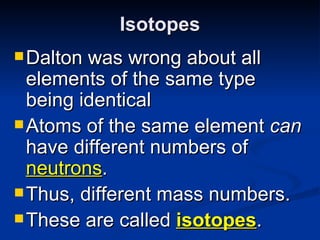
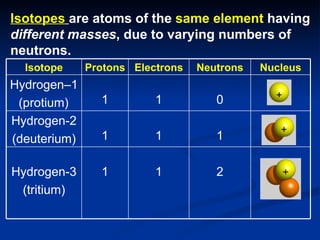
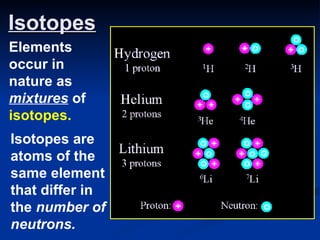
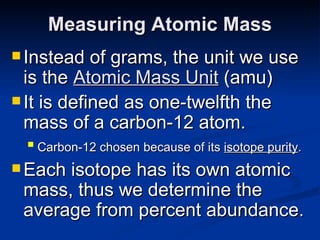
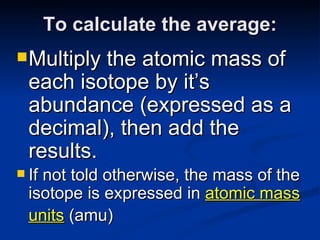
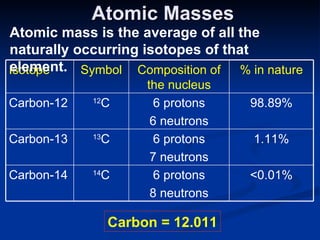
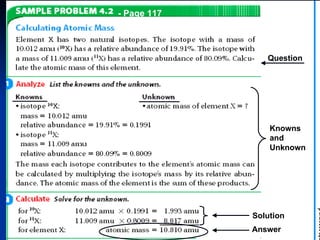
3) Isotopes are atoms of the same element that differ in number of neutrons. Atomic mass is an average that takes isotopic abundance into account. The periodic table organizes elements based on repeating atomic properties.