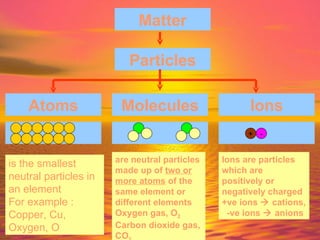
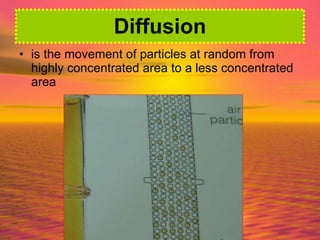
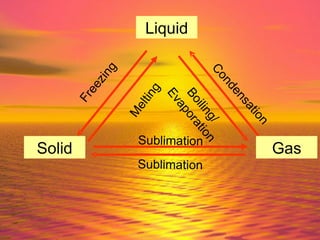
Matter is made up of particles, including atoms, molecules, and ions. Atoms are the smallest particles that make up elements. Matter exists in three states - solid, liquid, and gas - depending on how closely or loosely packed the particles are. In solids, particles vibrate in fixed positions; in liquids, they can move around but remain close together; and in gases, particles are far apart and move quickly in random directions. Phase changes between these states, such as melting, boiling, condensing, and sublimating, involve changes to the energy and movement of particles.