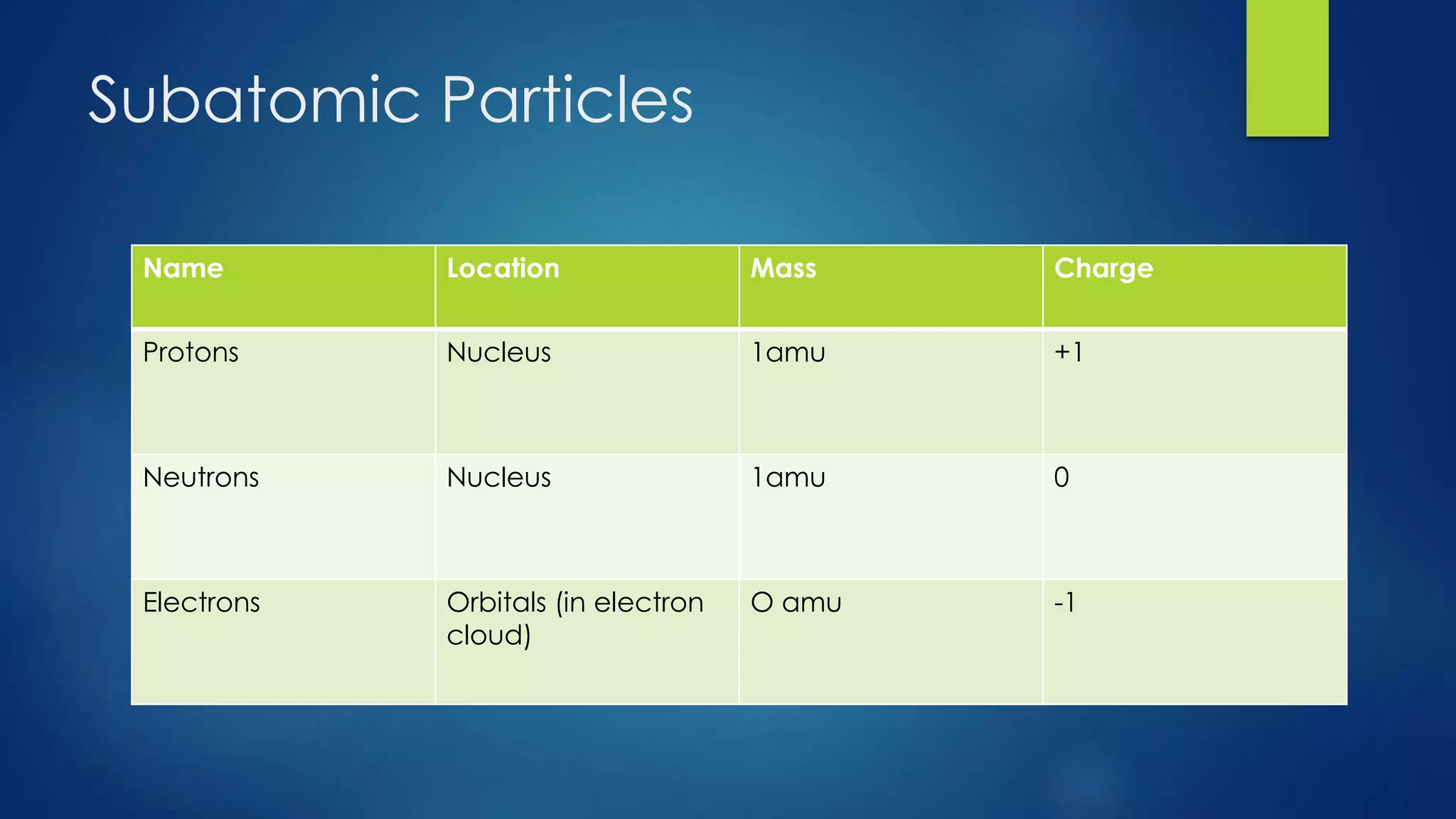
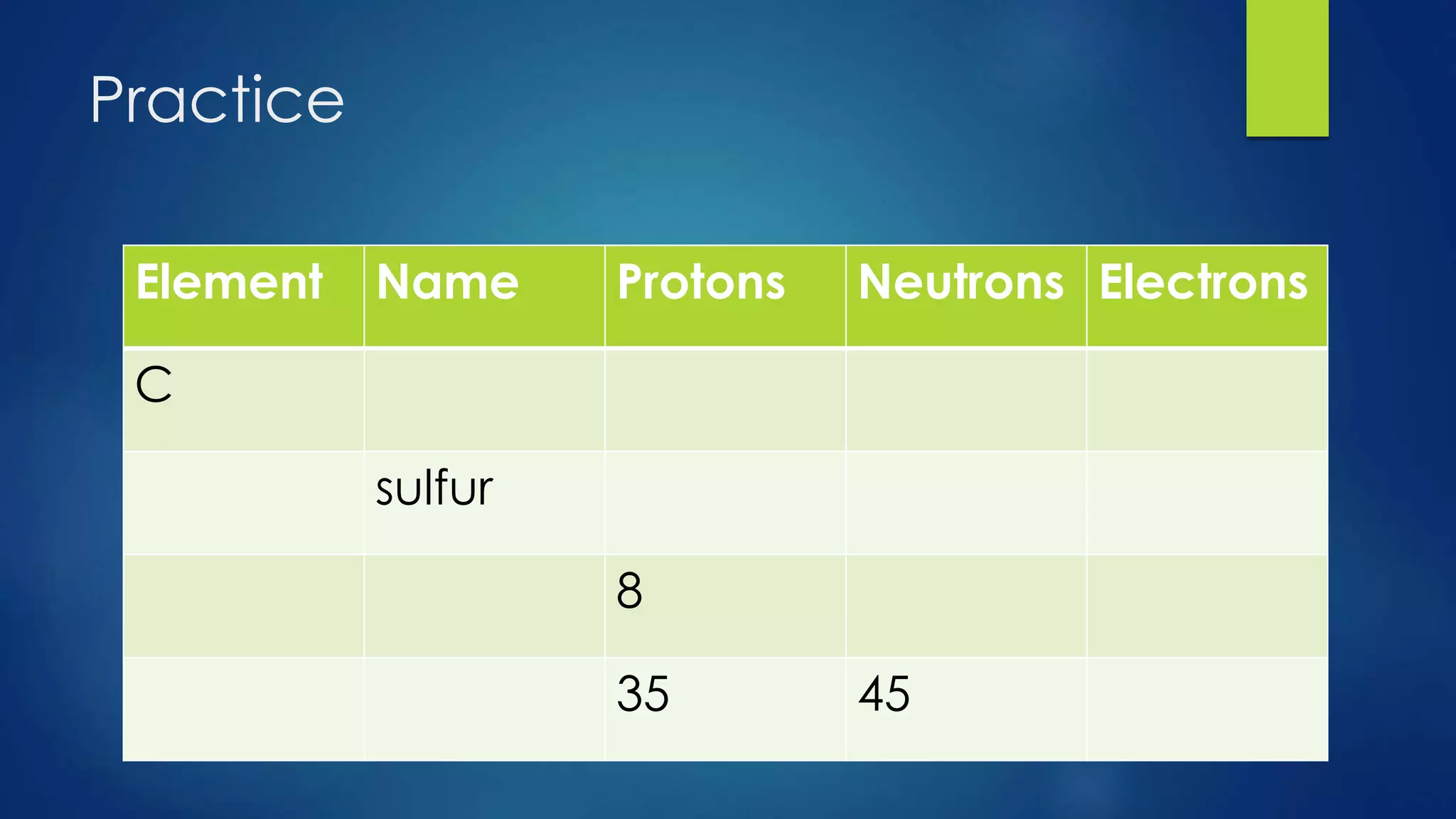
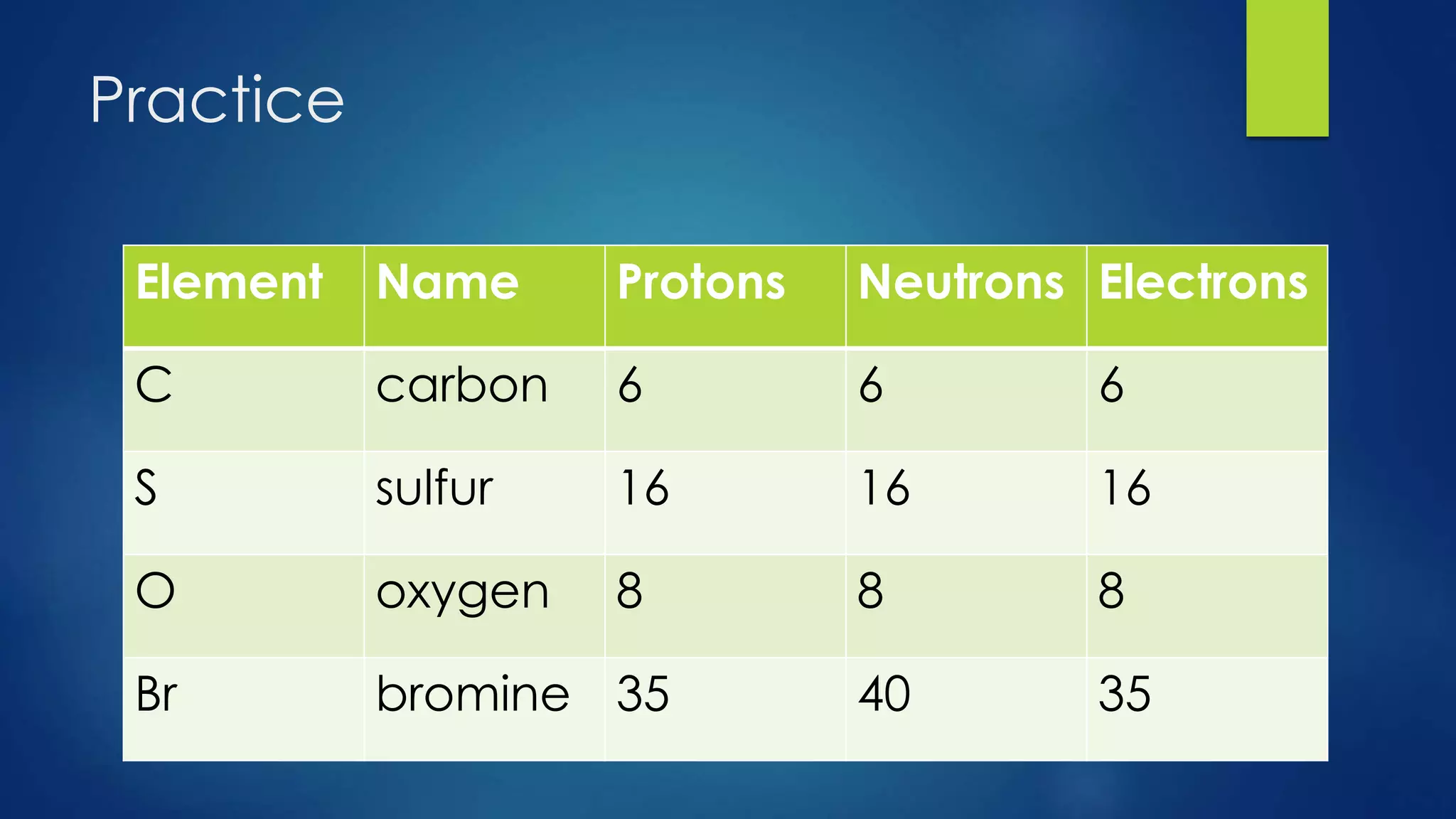
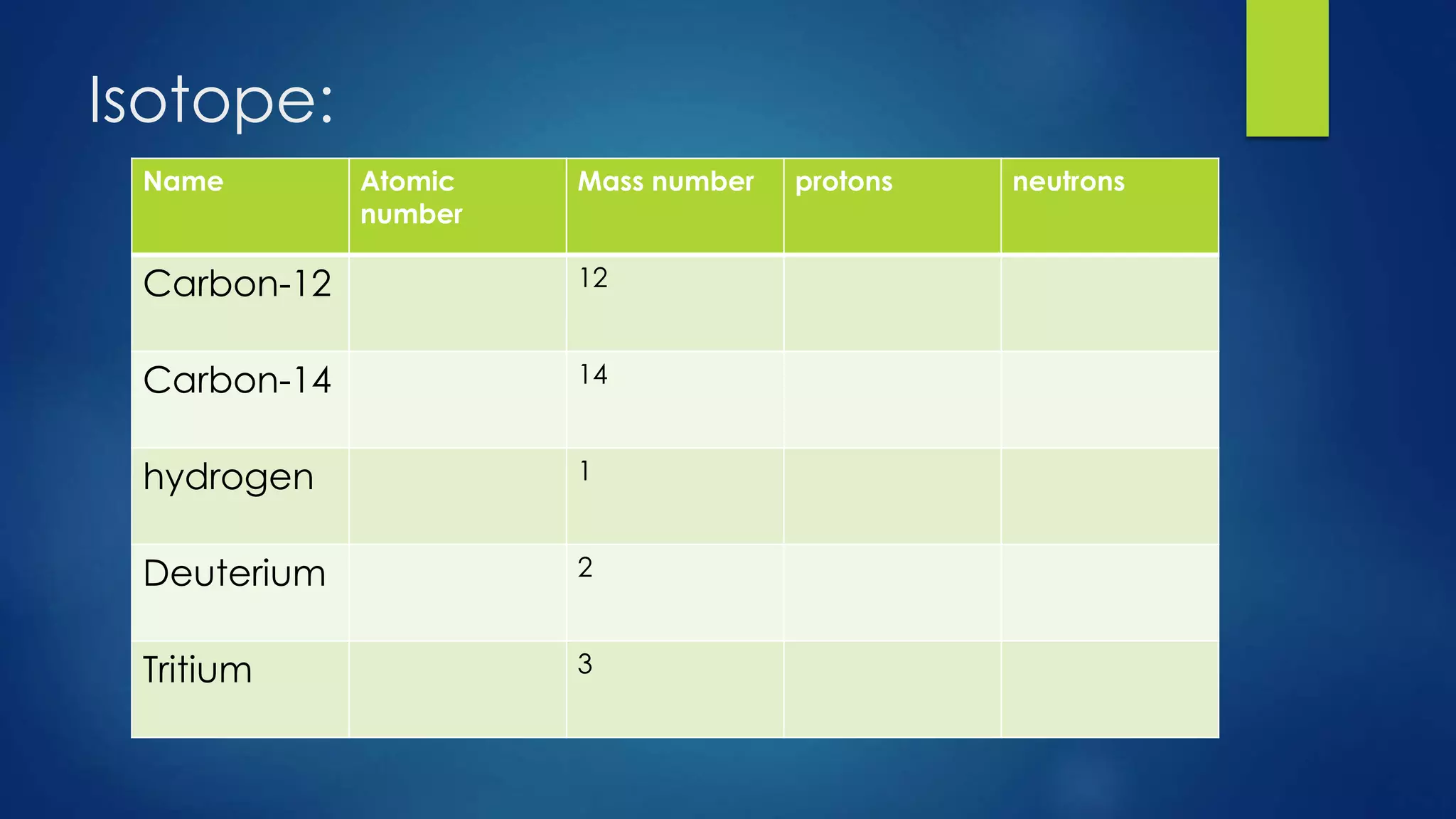
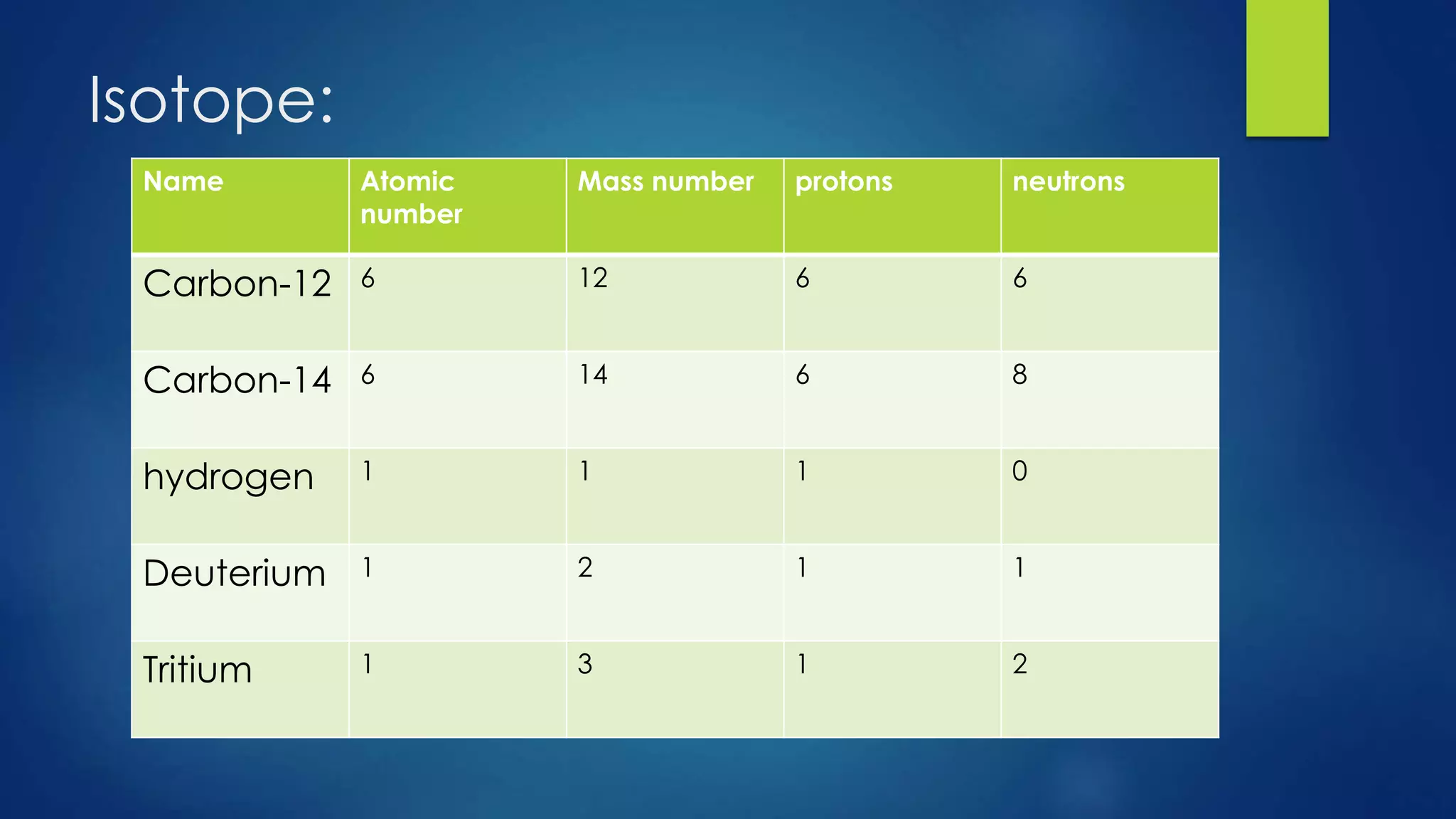
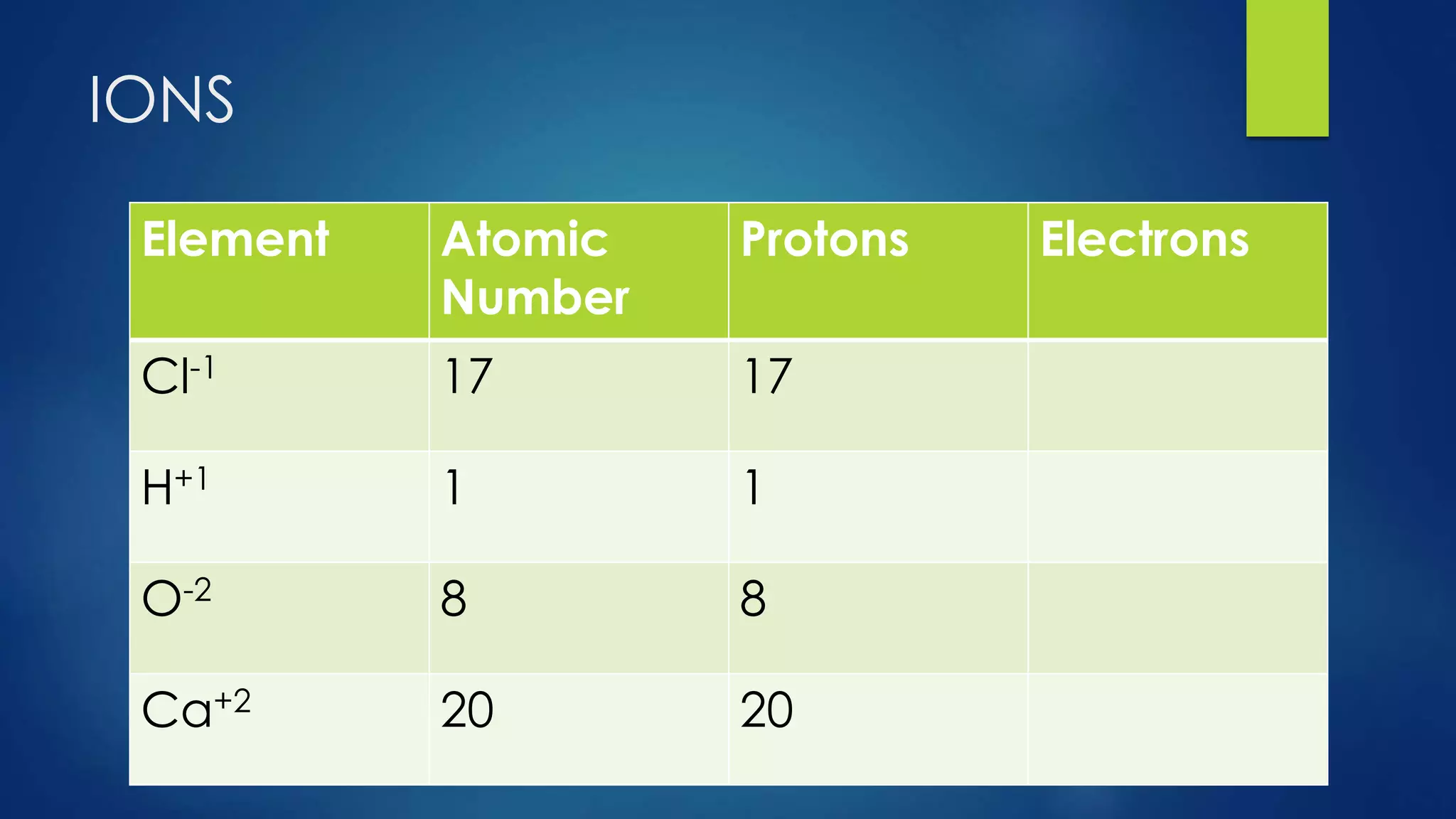
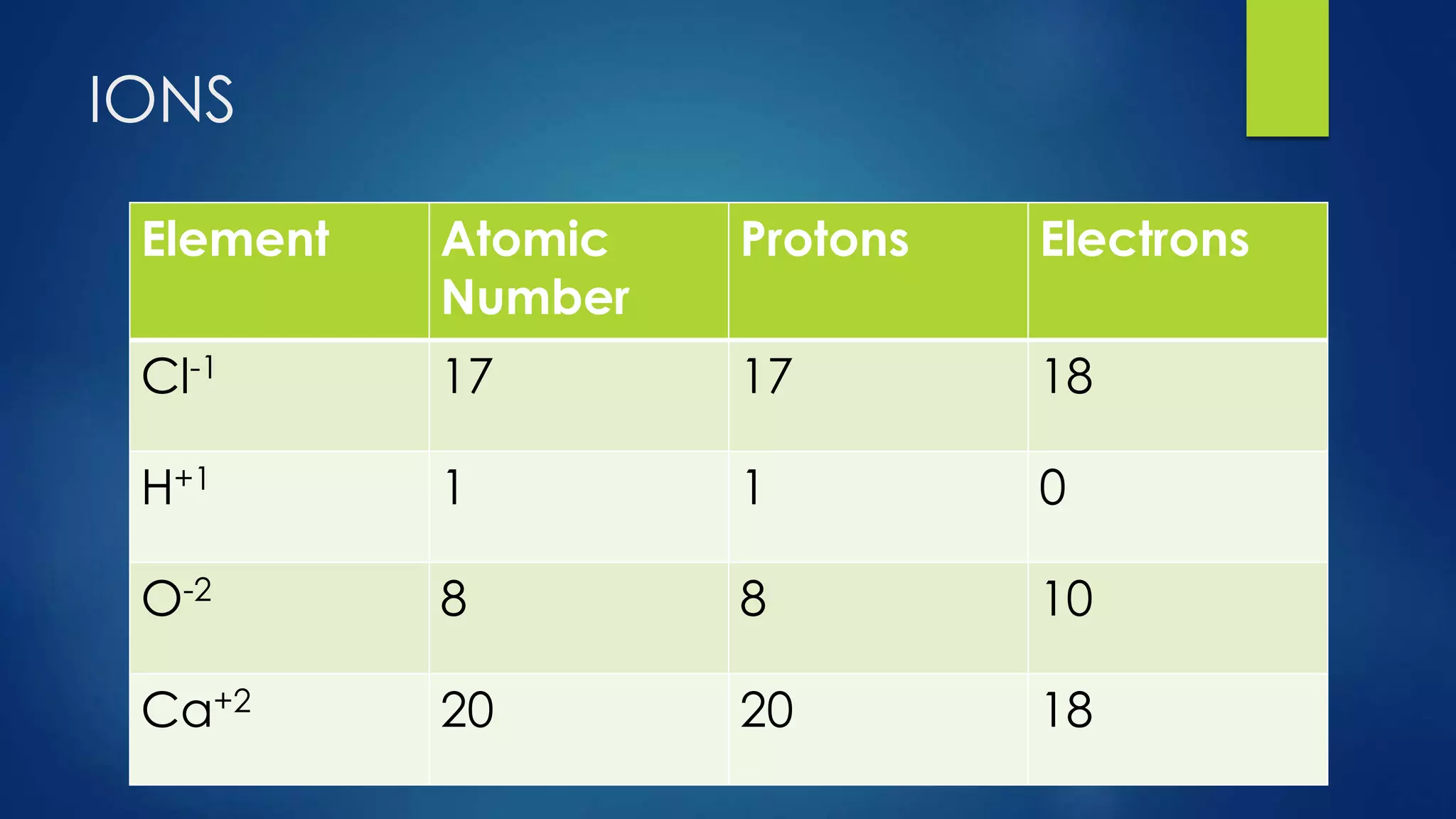
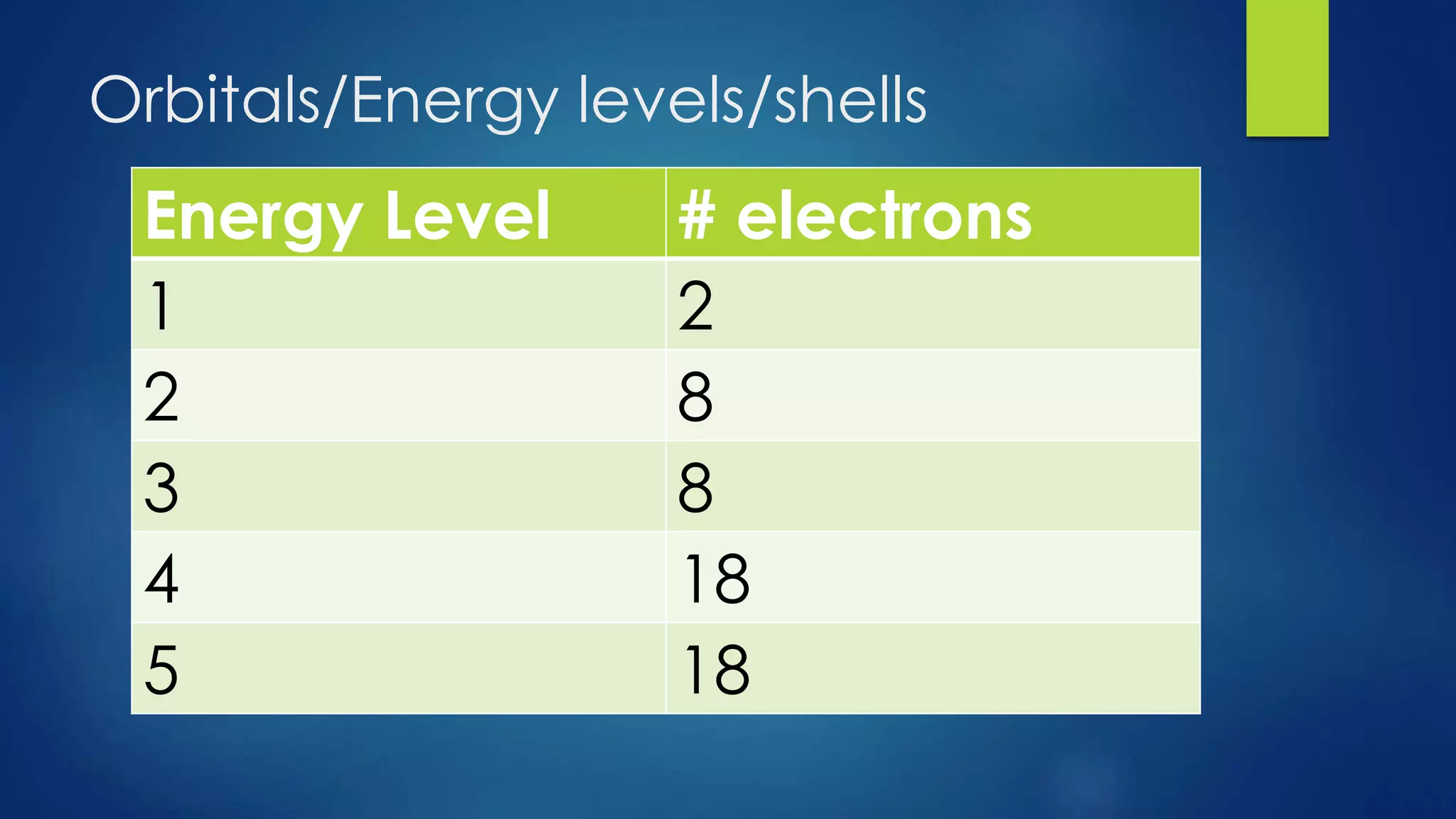
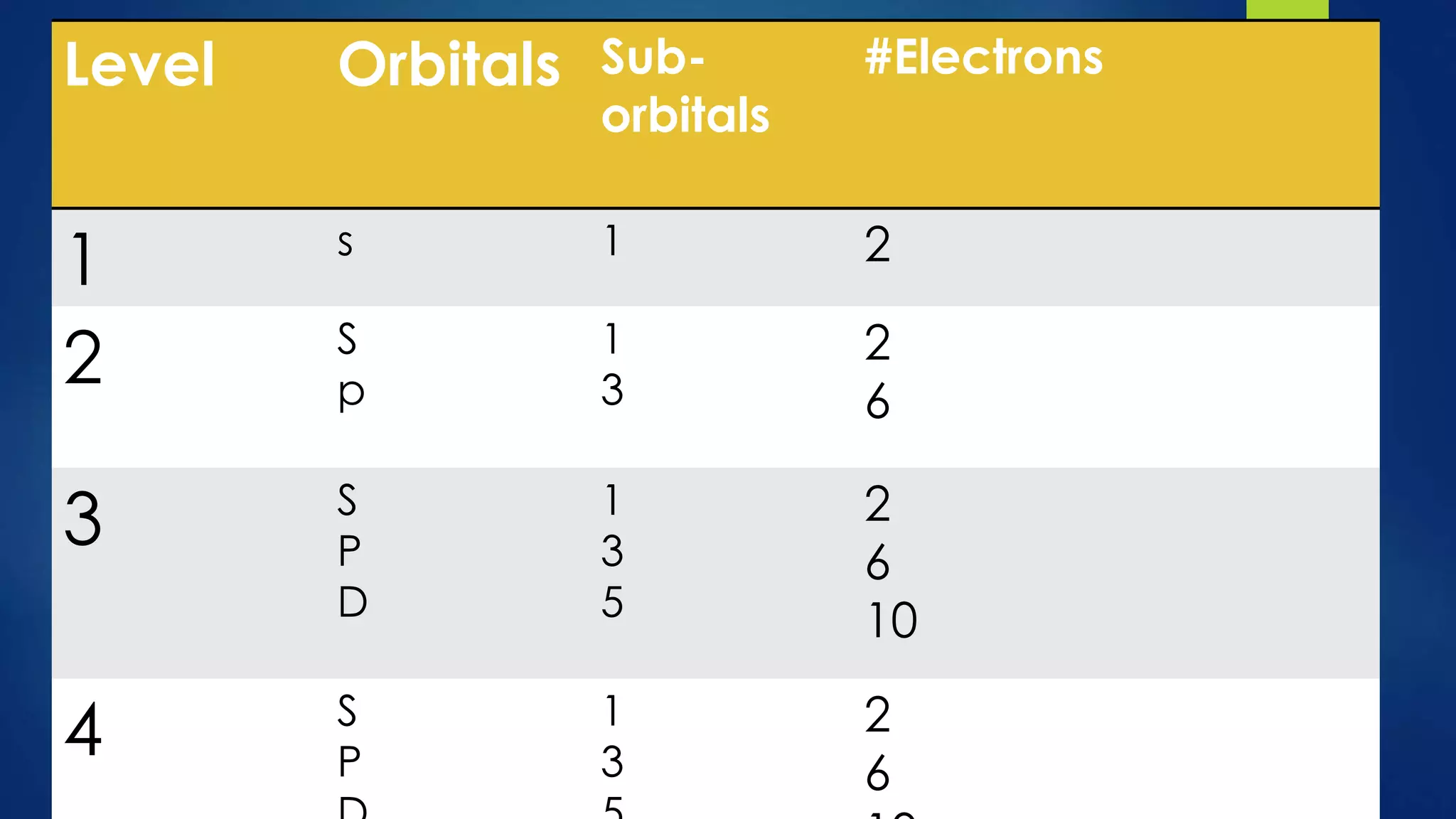
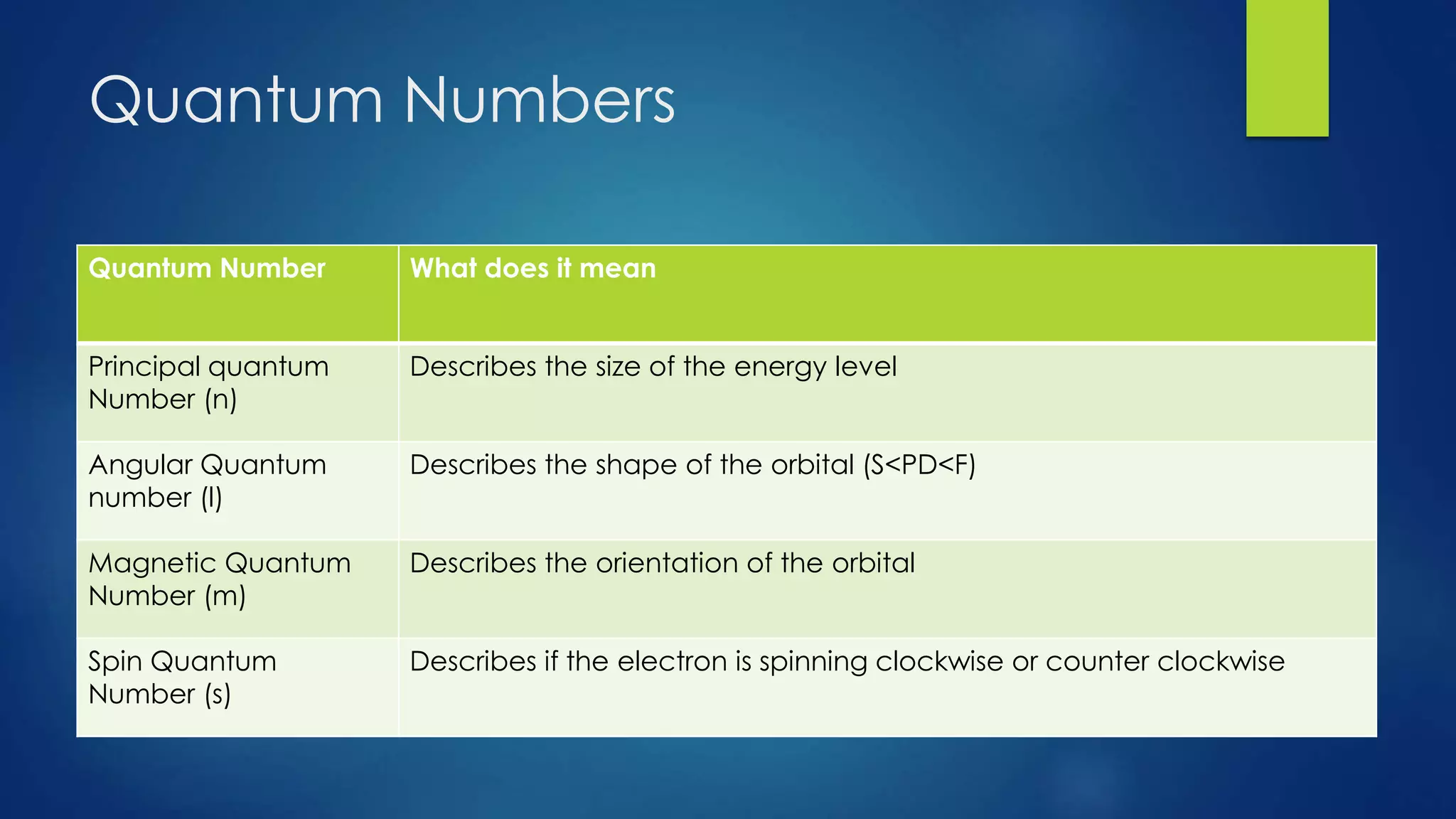
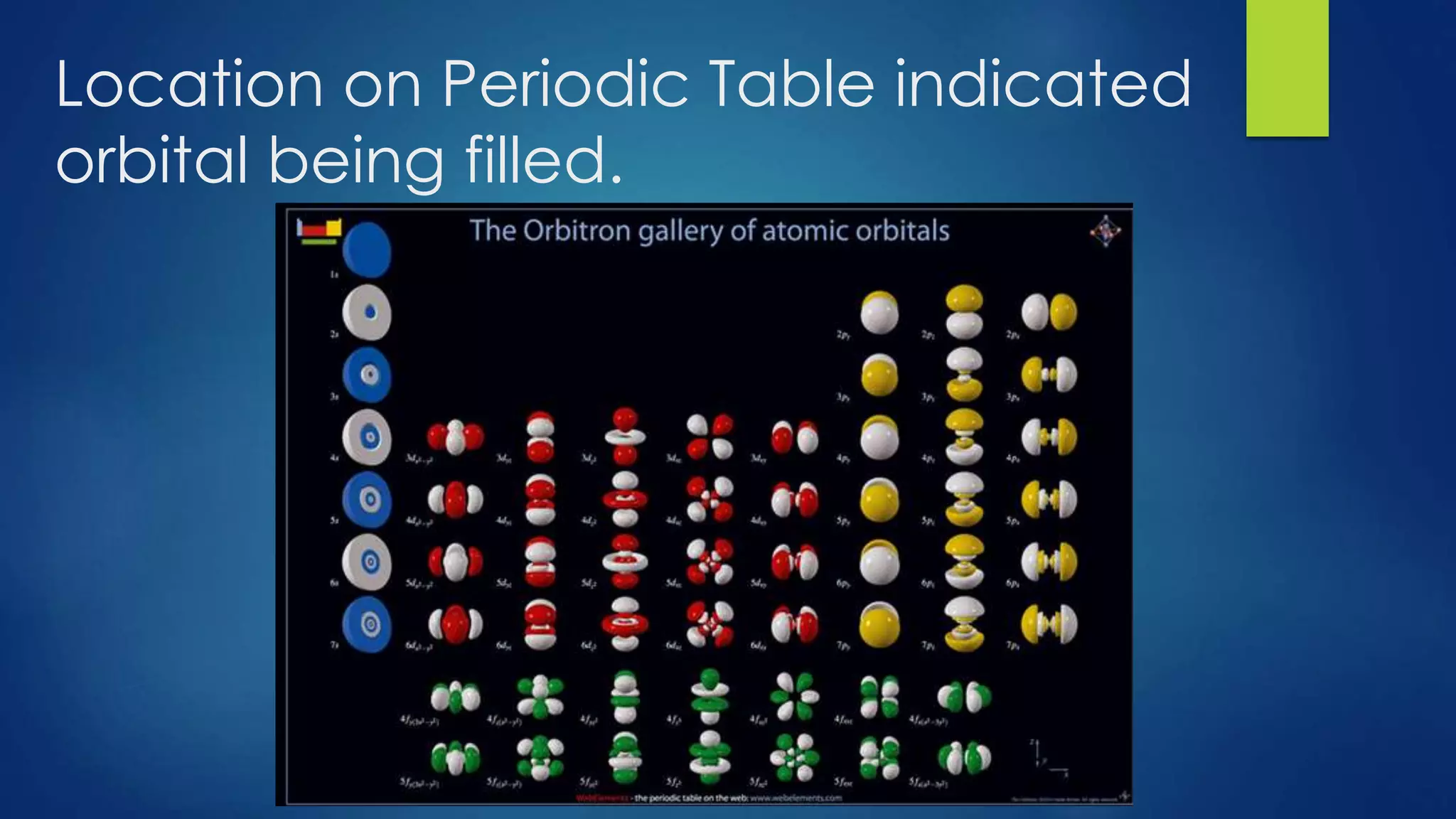
This document provides an overview of atomic structure and models of the atom. It discusses Dalton's atomic theory, subatomic particles including protons, neutrons, and electrons. Atoms are composed of a nucleus containing protons and neutrons, with electrons orbiting the nucleus. Elements differ based on their number of protons. Isotopes are versions of the same element that differ in their number of neutrons. The structure of atoms is further explained through electron configuration diagrams and quantum numbers that describe the location of electrons. Later atomic models such as the Bohr model and electron cloud model improved upon representing the structure and behavior of electrons.