Embed presentation
Download to read offline
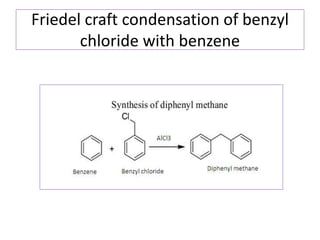
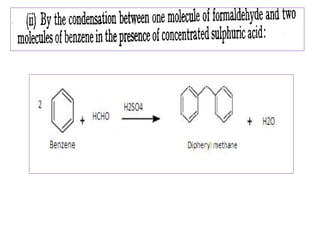
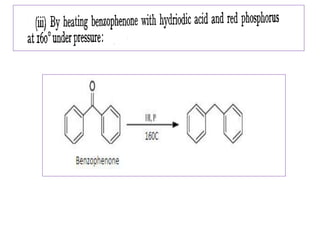
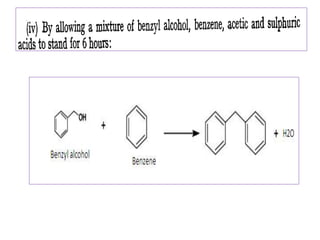
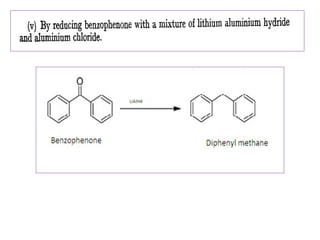
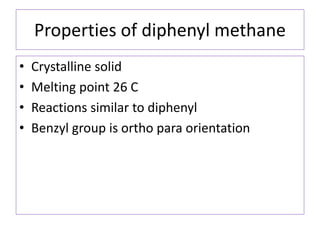
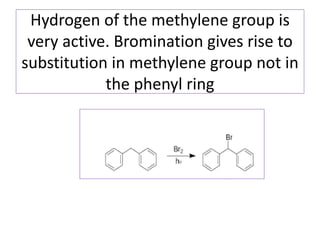
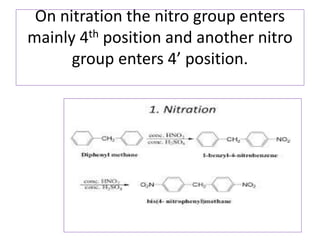
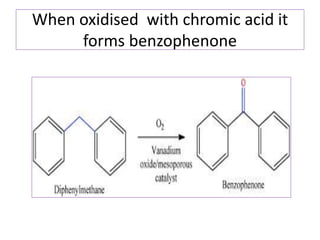
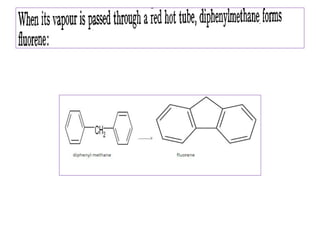
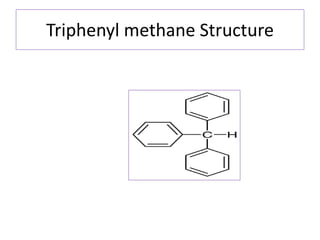



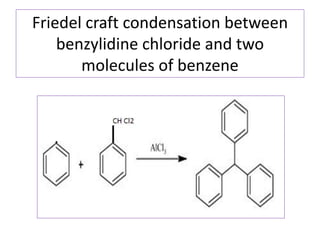
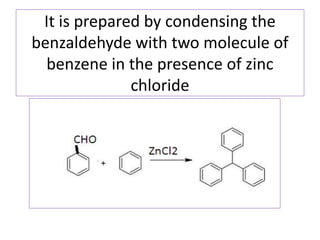
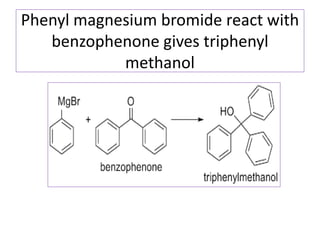
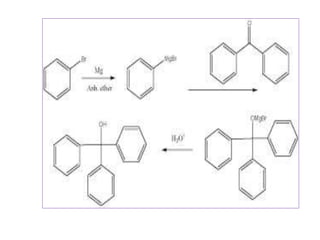
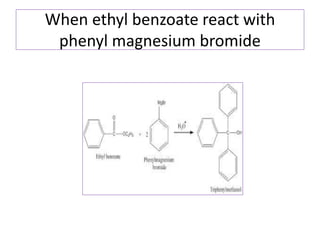

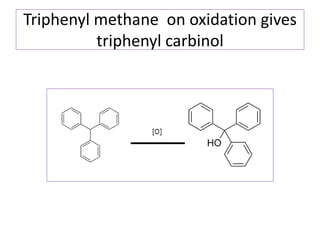
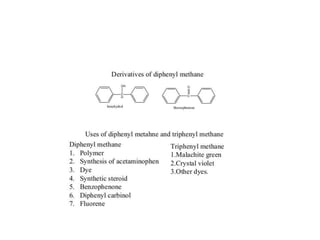
Diphenyl methane is produced through the Friedel craft condensation of benzyl chloride with benzene. It is a crystalline solid with a melting point of 26°C that undergoes substitutions in its methylene group rather than phenyl rings through reactions like bromination. Nitration adds nitro groups to the 4th and 4' positions. Oxidation with chromic acid forms benzophenone. Triphenyl methane is a colorless crystalline solid with a melting point of 93°C. It can be prepared through several Friedel craft condensations including between chloroform and three benzene molecules. It is the parent substance for triphenyl methane dyes.























