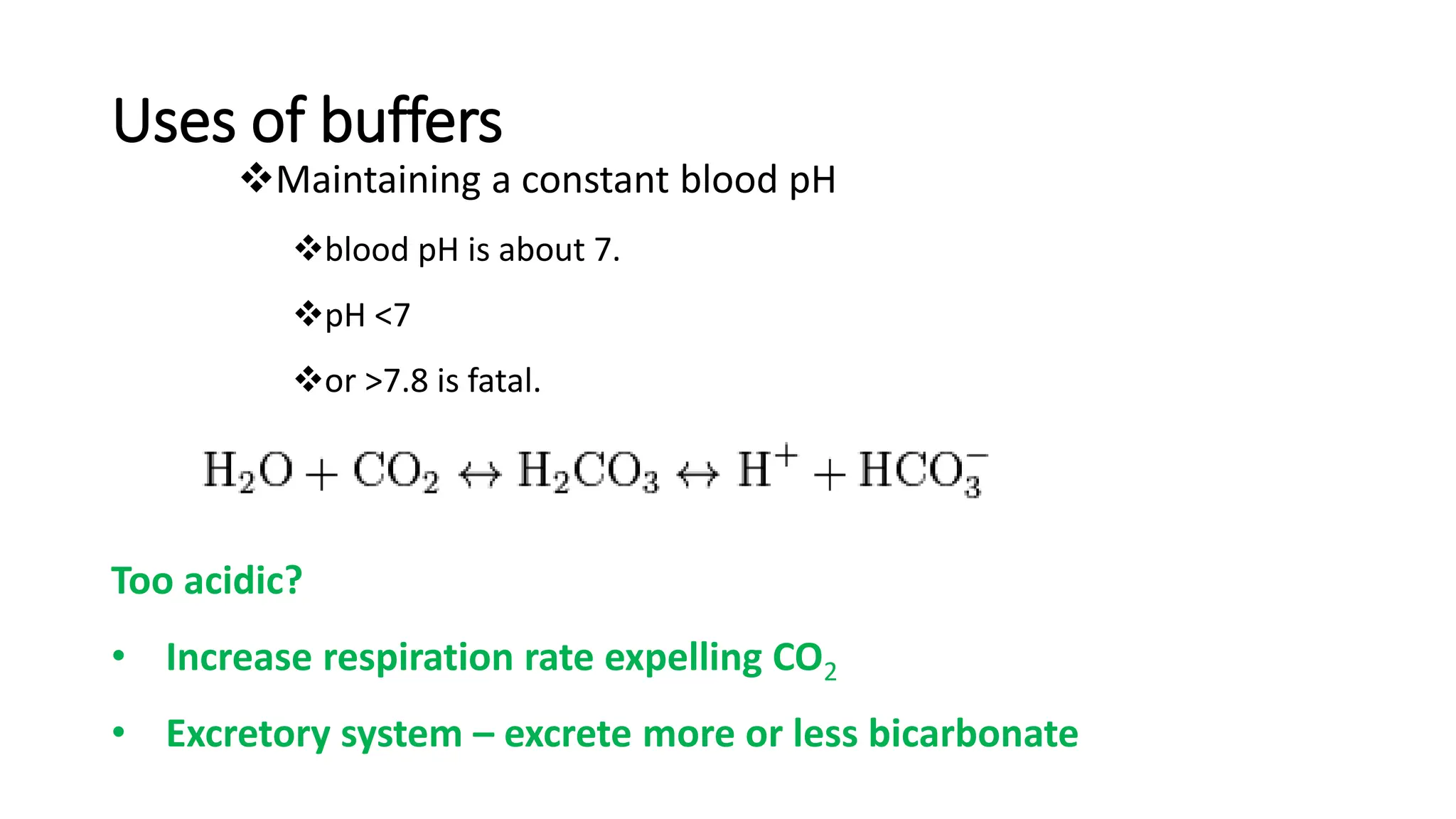
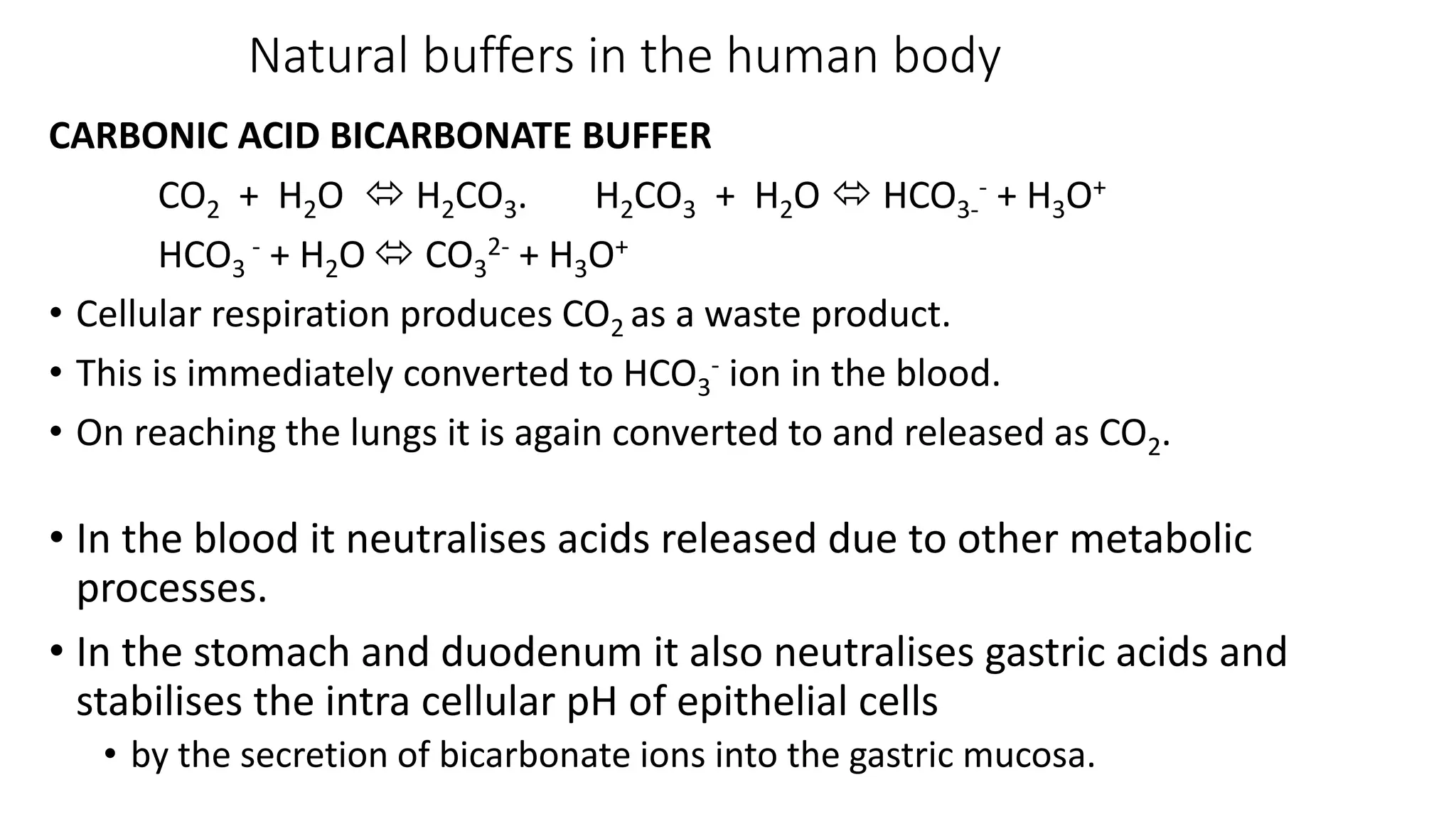
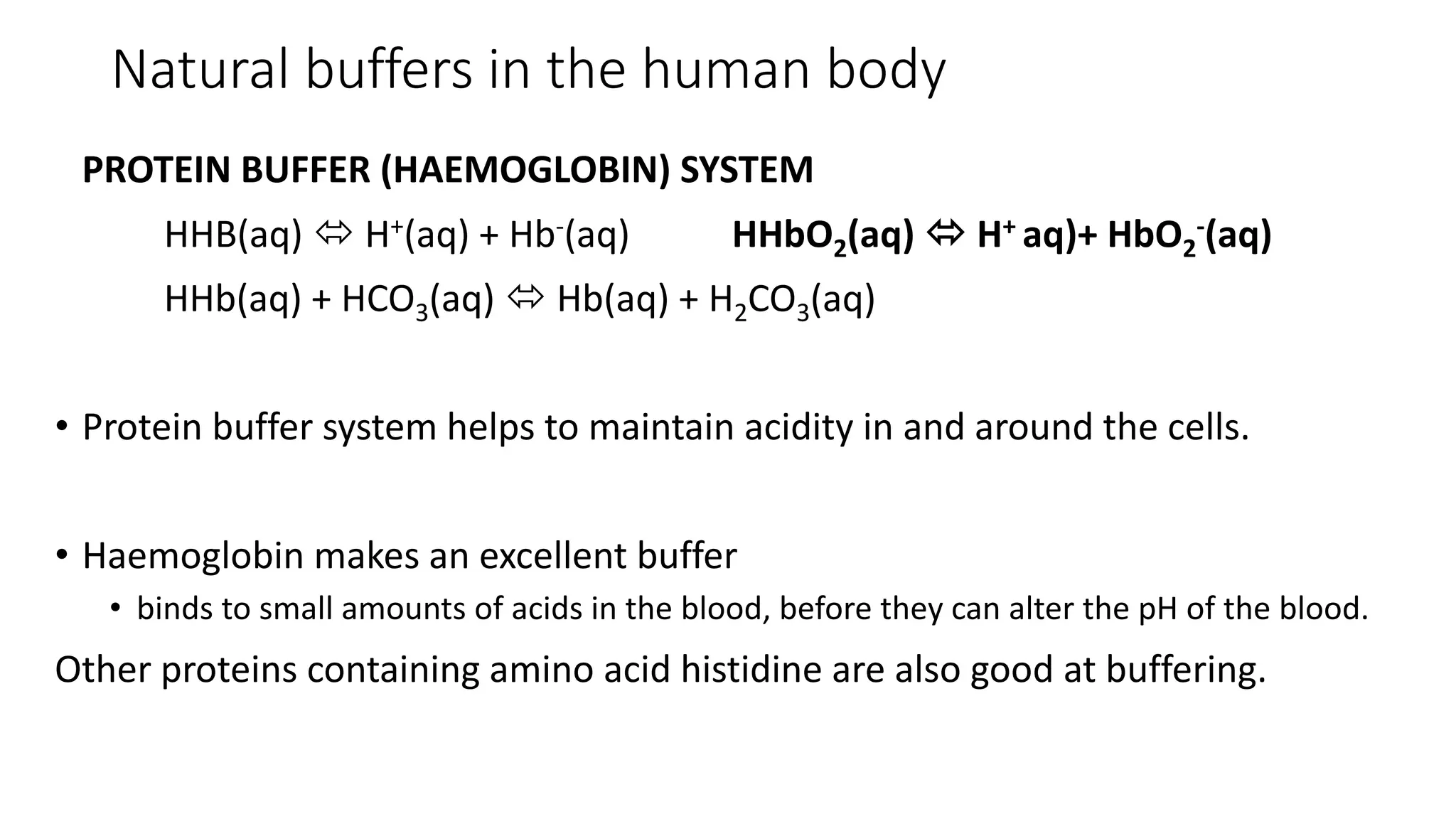
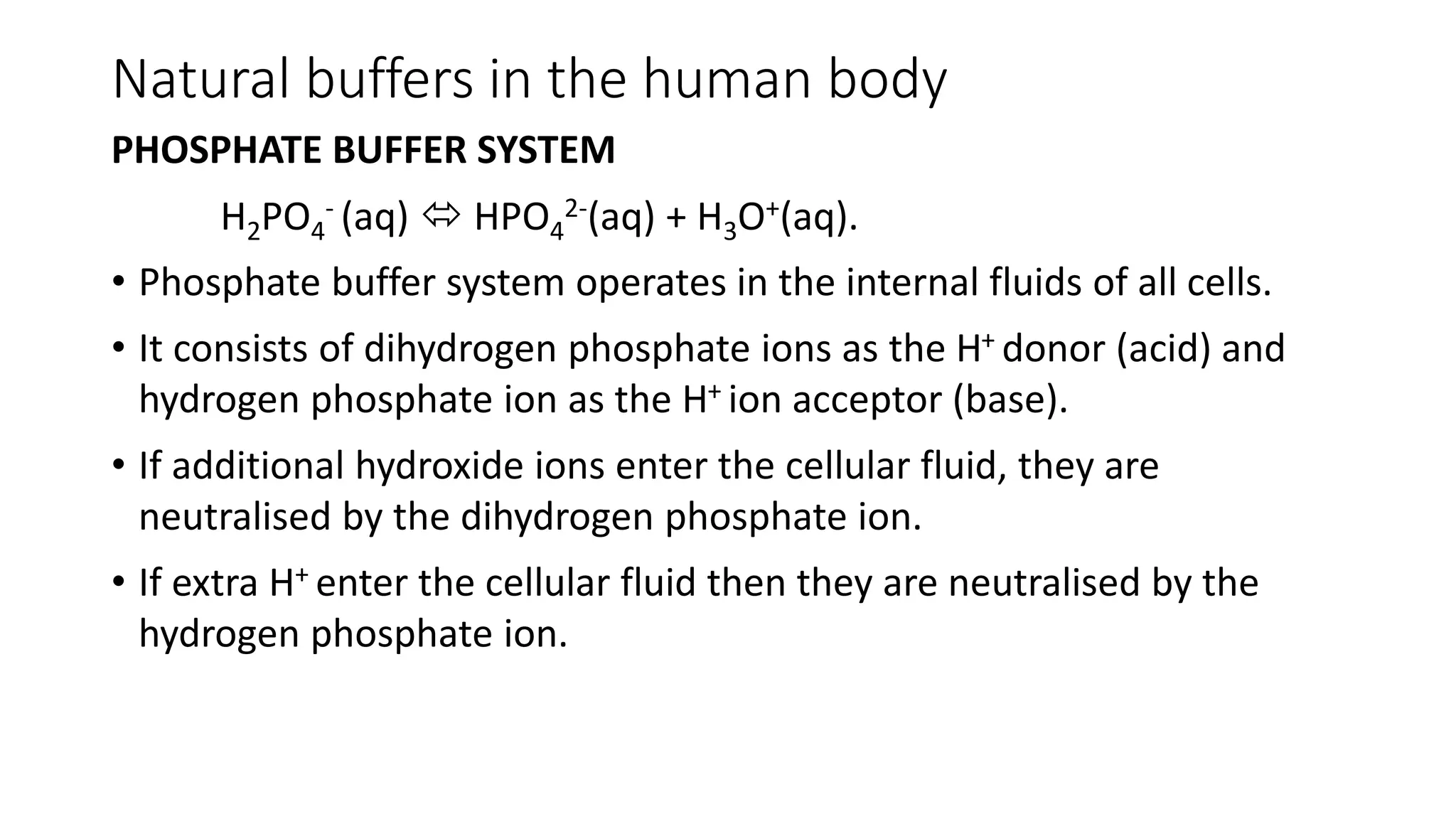
Buffer solutions are mixtures of weak acids and their conjugate bases that help maintain a stable pH when small amounts of acids or bases are added. They are essential in various applications, such as enzyme reactions, fermentation, and pharmaceuticals, and their effectiveness is determined by the concentration of the acid and base in the solution. The Henderson-Hasselbalch equation is used to calculate the pH of buffer solutions, and natural buffers in the human body play critical roles in maintaining physiological pH levels.




![How buffers work
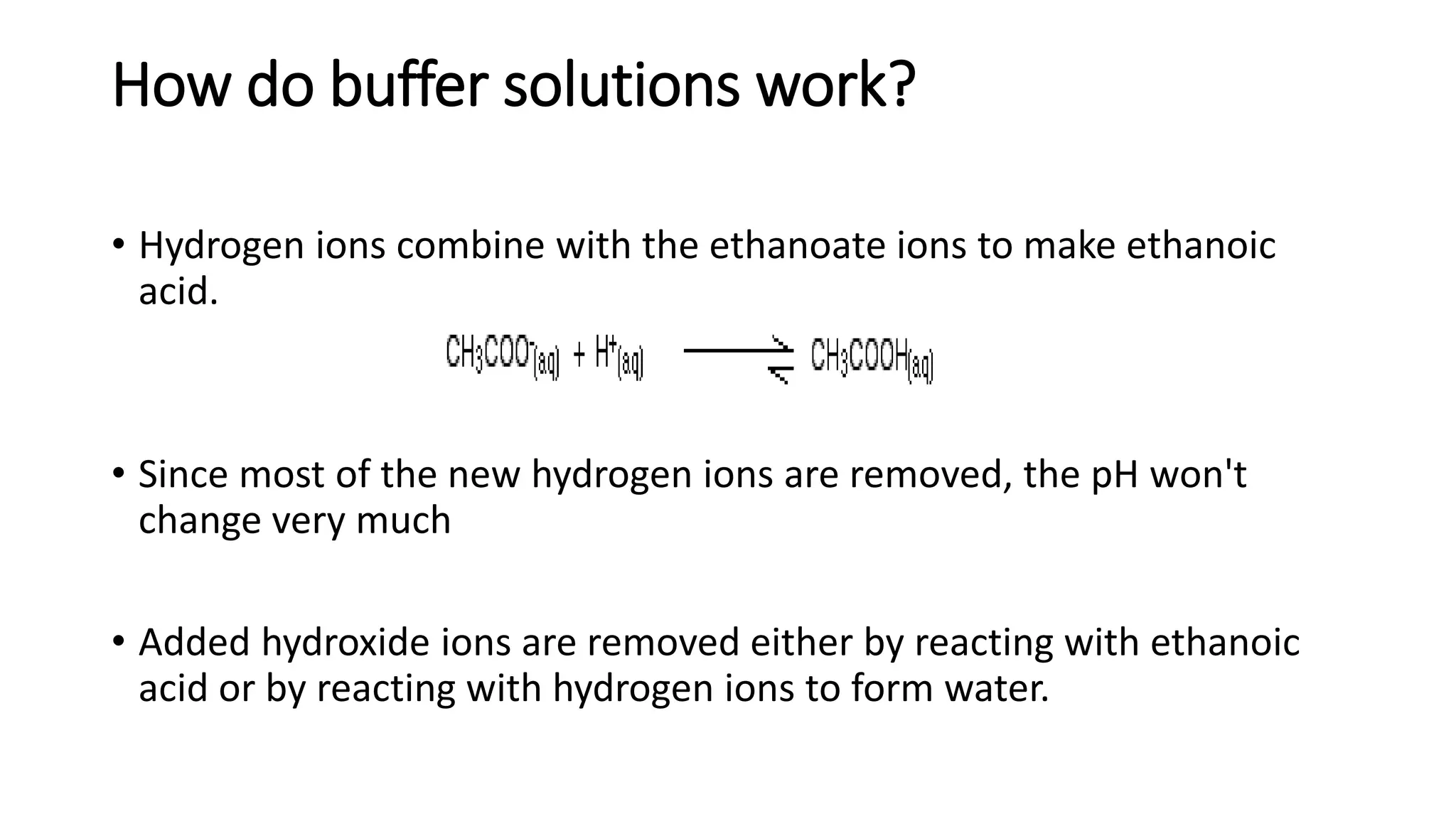
❖Equilibrium between acid and base.
❖Example: Acetate buffer
• CH3COOH CH3COO- + H+
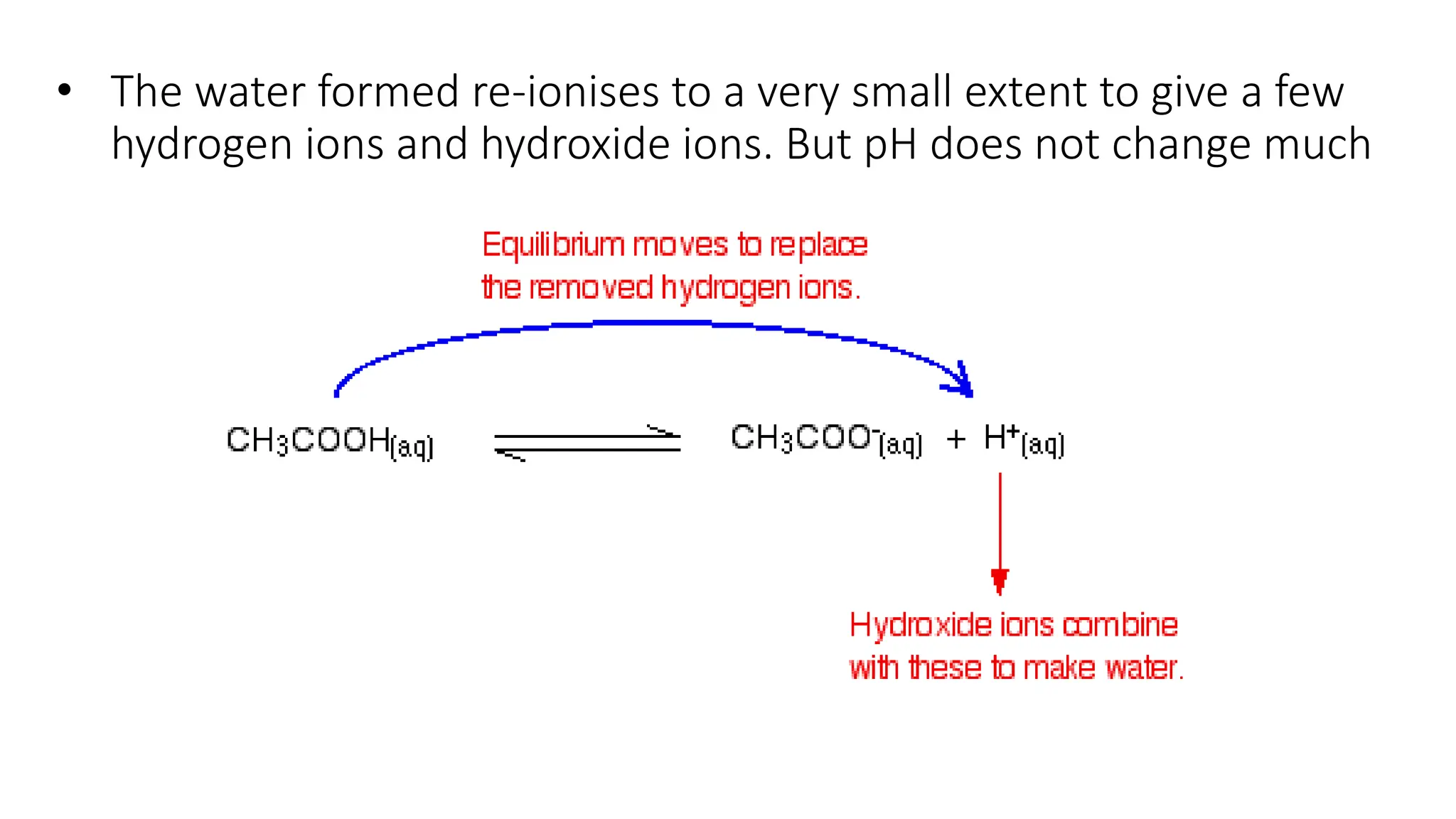
• If more H+ is added to this solution, it simply shifts the equilibrium
to the left, absorbing H+, so the [H+] remains unchanged.
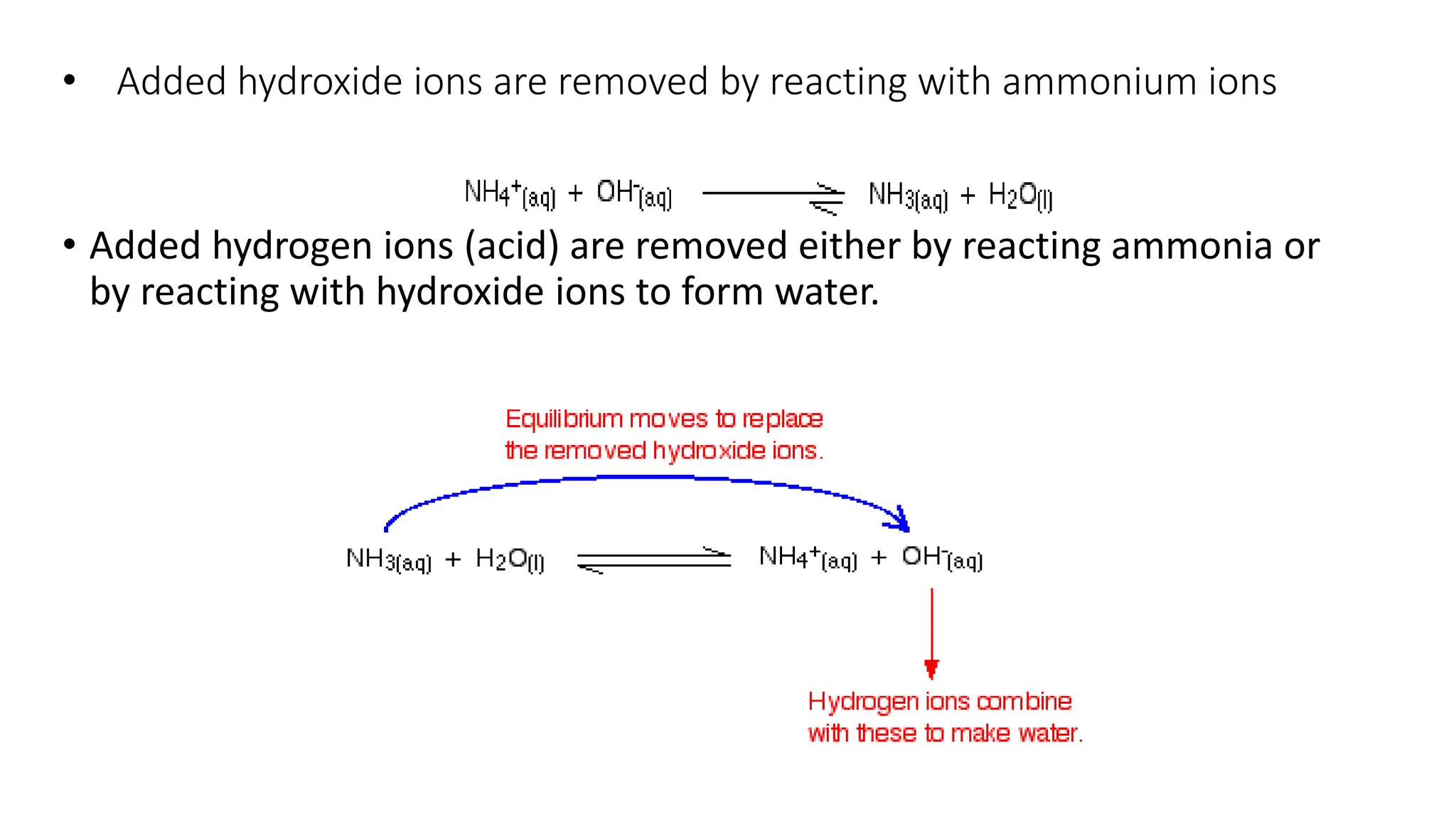
• If H+ is removed (e.g. by adding OH-) then the equilibrium shifts to
the right, releasing H+ to keep the pH constant](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-10-2048.jpg)


![Chemistry of buffers
Equilibrium constant/Dissociation constant (Ka)
❖Ka = equilibrium constant for H+ ion transfer is also described as the dissociation
constant (the tendancy of an acid to dissociate).
AH → A- (base conjugate) + H+
Ka = [A-] [H+]/ [AH] = [base] [H+] / [acid]
❖Weak acids have low values… contribute few H+ ions…
❖Because we are usually dealing with very small concentrations, log values are
used…
❖The log constant =](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-21-2048.jpg)
![Chemistry of buffers
❖Since pK is the negative log of K, weak acids have high values … (-2 –
12).
❖HCl = -9.3 – very low ~complete dissociation
• First rearrange the first equation and solve for [H+]
• [H+] = Ka x [acid]/[base]
❖Then take the log of both sides
• log10[H+] = log10Ka + log10 [acid]/[base]
-pH -pKa](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-22-2048.jpg)
![Chemistry of buffers
❖-pH = -pKa + log10 [acid]/[base]
❖Multiply both sides by –1 to get the Henderson-Hasselbach equation
• pH = pKa - log10 [acid]/[base]](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-23-2048.jpg)
![Chemistry of buffers
❖What happens when the concentration of the acid and base are equal?
• Example: Prepare a buffer with 0.10M acetic acid and 0.10M acetate
• pH = pKa - log10 [acid]/[base]
• pH = pKa - log10 [0.10]/[0.10]
• pH=pKa
• Thus, the pH where equal concentrations of acid and base are
present is defined as the pKa
❖If there is more of the conjugate base in the solution than acid, for
example, then pH > pKa. Conversely, if there is more acid than conjugate
base in solution, then pH < pKa.
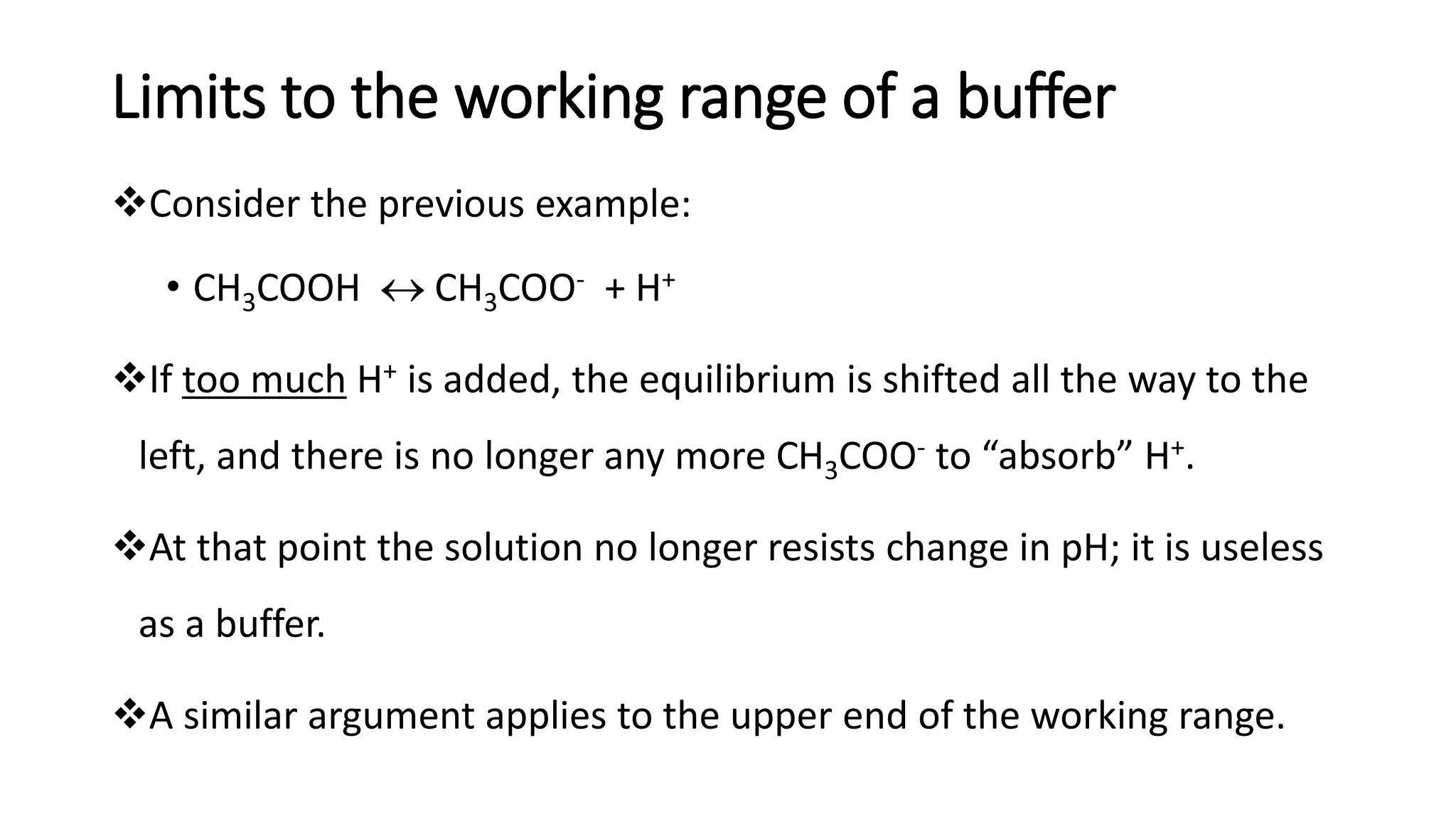
❖A buffer works most effectively at pH values that are + 1 pH unit from the
pKa (the buffer range).](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-24-2048.jpg)
![pH of a buffer solution
• The pH of a buffer system is given by the Henderson-Hasselbalch equation:
• for a weak acid and its salt:
• for a weak base and its salt:
• where [salt], [acid] and [base] are the molar concentrations of salt, acid and
base.](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-26-2048.jpg)
![Preparation of a buffer solution of a given pH: e.g. prepare 2 litres of solution
of buffer pH 7.4
• Choose weak acid whose pKa is close to desired pH, for example H2PO4
_; Ka =
6.2 x 10-8, pKa = 7.21
• Use Henderson –Hasselbalch equation to calculate amounts of base and acid.
Acid base (salt)
H2PO4
- + H2O → HPO4
2_ + H3O+
Ka = 6.2 x 10-8. pKa = 7.21
7.4 = 7.21 + log[base]/[acid]
7.4 -7.21 = log[base]/[acid]
0.19 = log[base]/[acid]
[base]/[acid]= 100.19 = 1.5; i.e. base to acid ratio of 1.5](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-27-2048.jpg)
![Preparation of 2L of pH 7.4 buffer solution
• Choose [base] to be 1.5M, then [acid] will be 1M to give a ratio of 1.5.
• Base = HPO4
2_; choose cation of Na, i.e. Na2HPO4; molar mass = 143g
• Acid = H2PO4
-; with cation of Na, i.e. NaH2PO4; molar mass = 120g
• For one litre of solution mass of base (Na2HPO4) is 1.5 x 143g = 214.5g and mass of acid
(NaH2PO4) is 1 x 120g = 120g.
• For two litres of buffer solution multiply the masses for 1 litre by 2.
• Mass of base (Na2HPO4) = 2 x 214.5g = 429g; mass of acid (NaH2PO4) = 2 x 120g = 240g
• Weigh 429g of Na2HPO4 and 240g of NaH2PO4 and dissolve them in 2 litres of distilled water and
mix well.](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-28-2048.jpg)
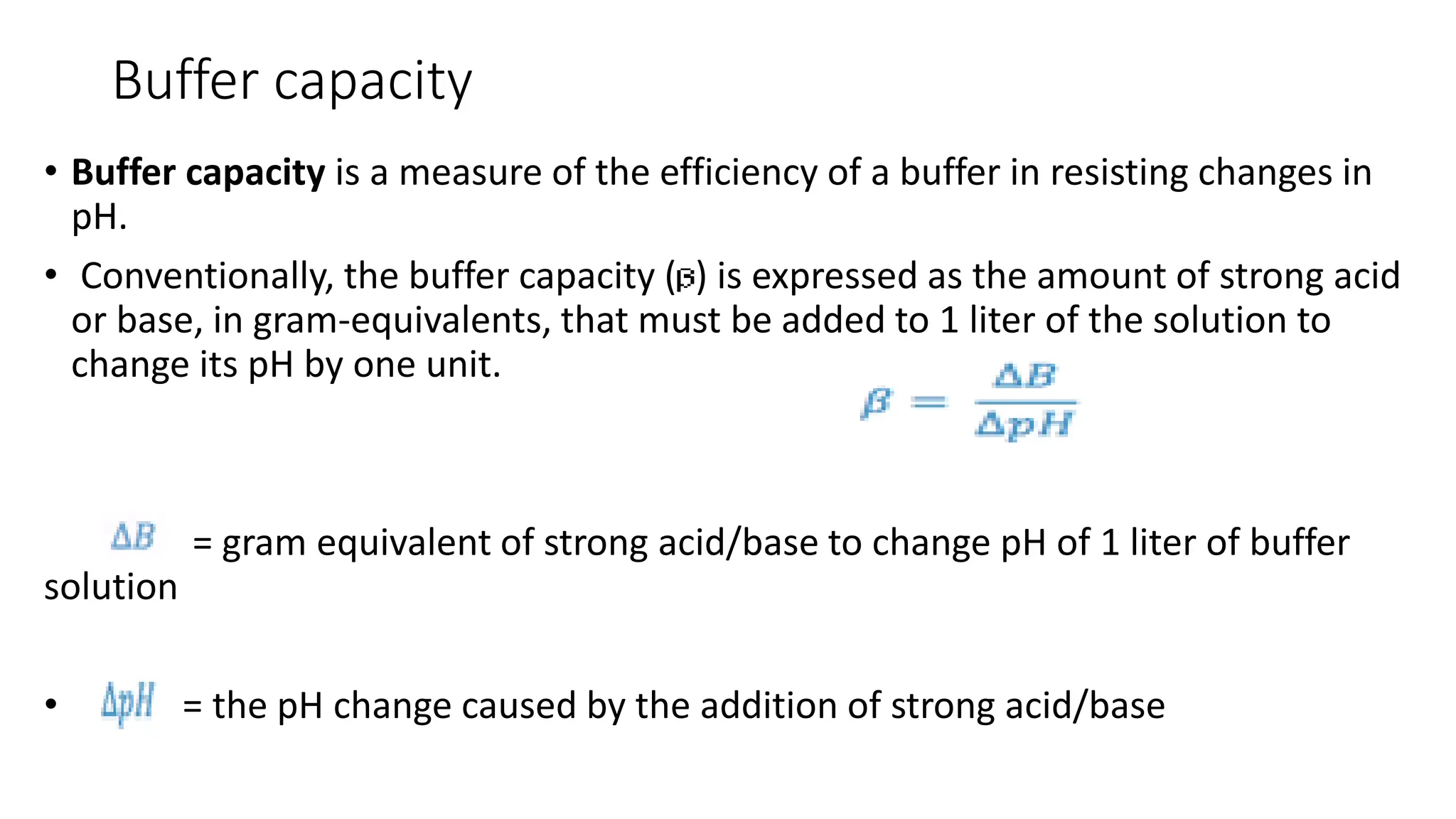
![Buffer capacity
• In practice, smaller pH changes are measured and the buffer capacity is
quantitatively expressed as the ratio of acid or base added to the change in pH
produced
• The buffer capacity depends essentially on 2 factors:
• Ratio of the salt to the acid or base: The buffer capacity is optimal when the ratio is
1:1; that is, when pH = pKa
• Total buffer concentration: For example, it will take more acid or base to deplete a
0.5 M buffer than a 0.05 M buffer.
• The relationship between buffer capacity and buffer concentrations is given by the
Van Slyke equation:
where C = the total buffer concentration (i.e. the sum of the molar concentrations
of acid and salt).
• Concentration of acid and base should be >>> [H3O+]](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-30-2048.jpg)



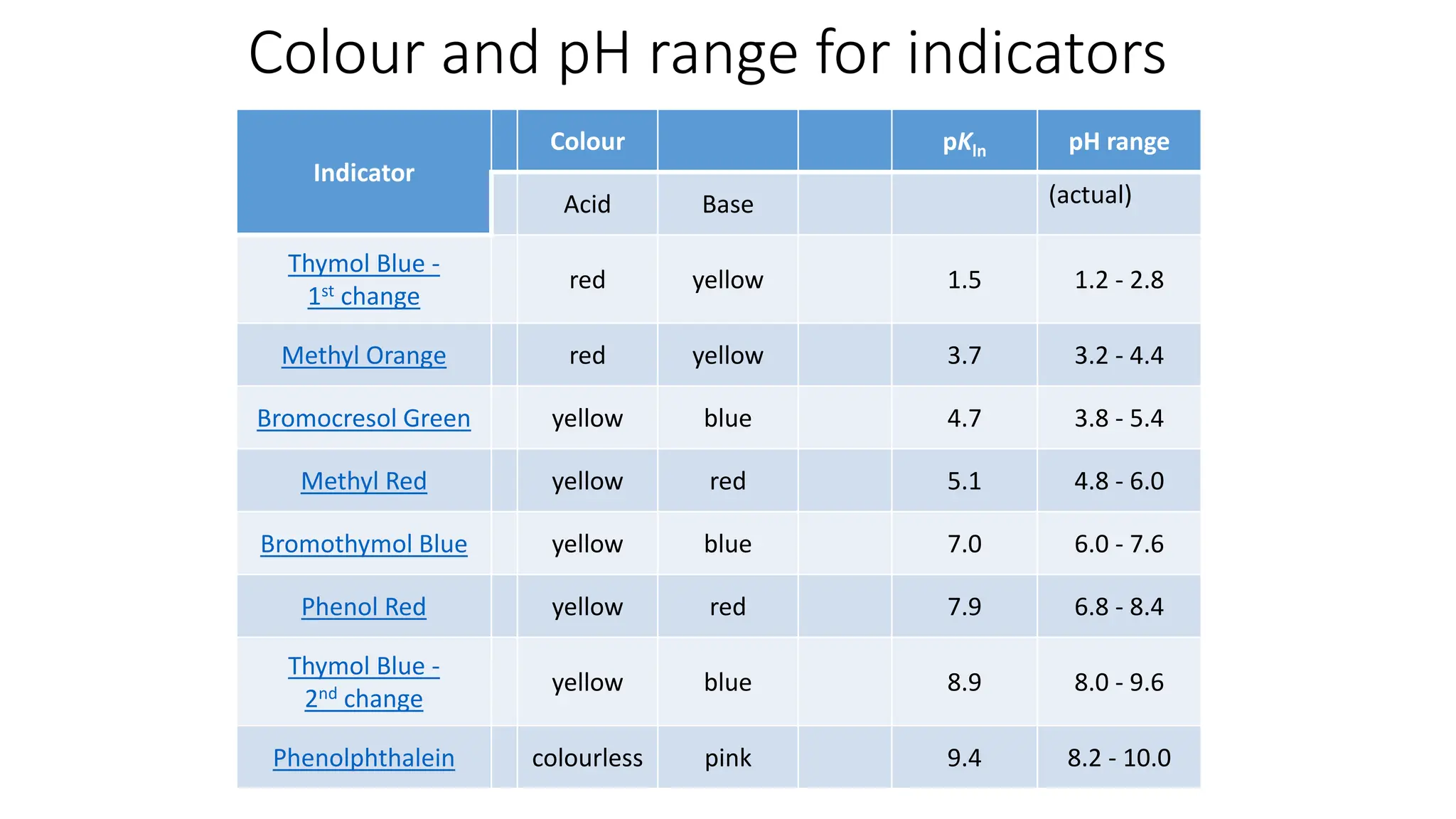
![pH range for indicator
• pH = pKa + log10[In−] / [Hin]
• For the reaction mixture to impart colour of HIn with confidence, [In-(aq)]/
[HIn(aq)] has to be ≤ 1/10 .
• For the reaction mixture to impart colour In- with confidence, [In-(aq)]/
[HIn(aq)] has to be ≥ 10.
• In other words, for the reaction mixture to impart colour of HIn with
confidence, pH value of the solution should be pKa - 1 or lower, and
• For the reaction mixture to impart colour of In- with confidence, pH value of
the solution should be pKa +1 or higher.
• Hence pH range of an indicator = pKa ± 1.](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-38-2048.jpg)
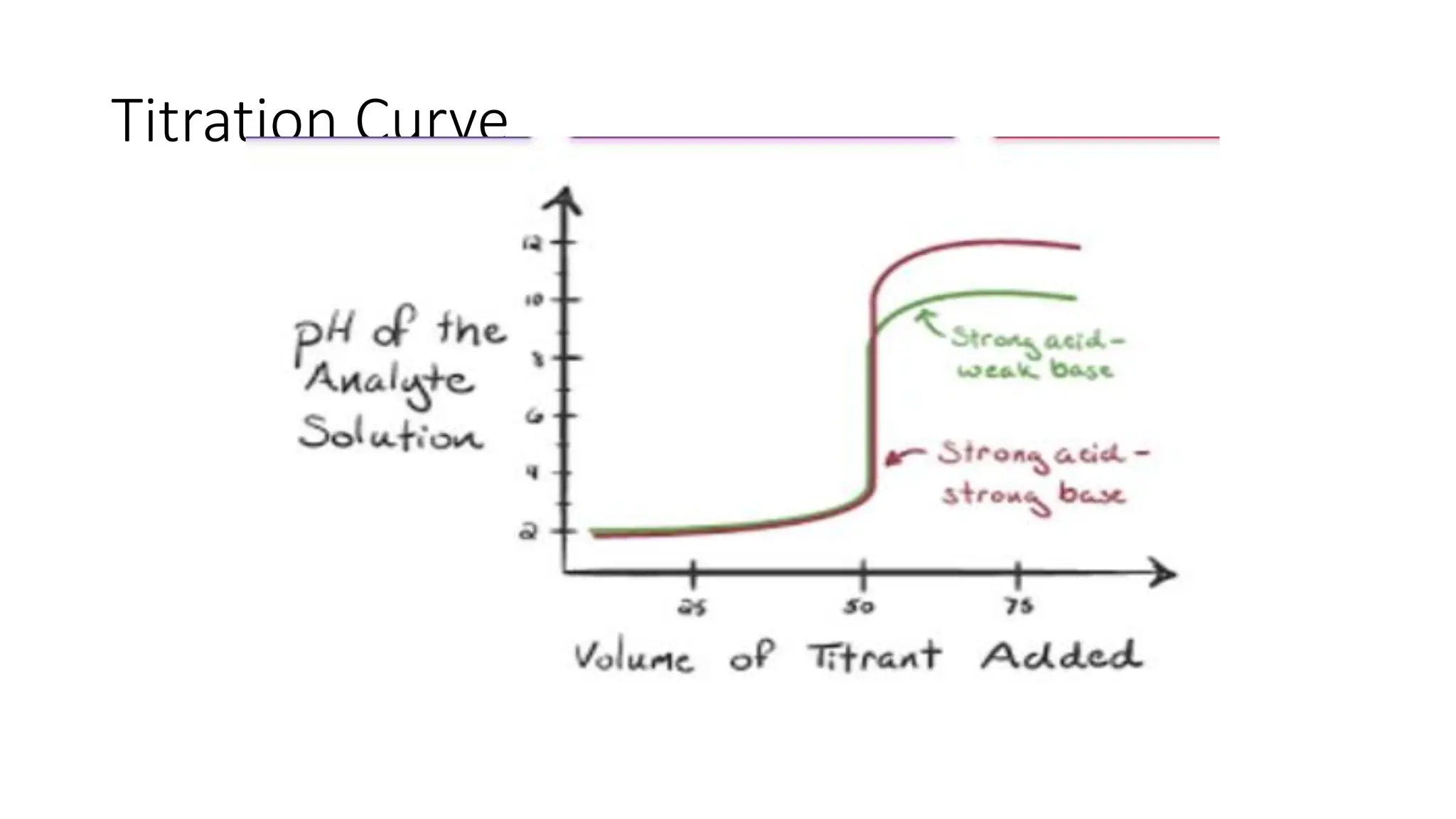
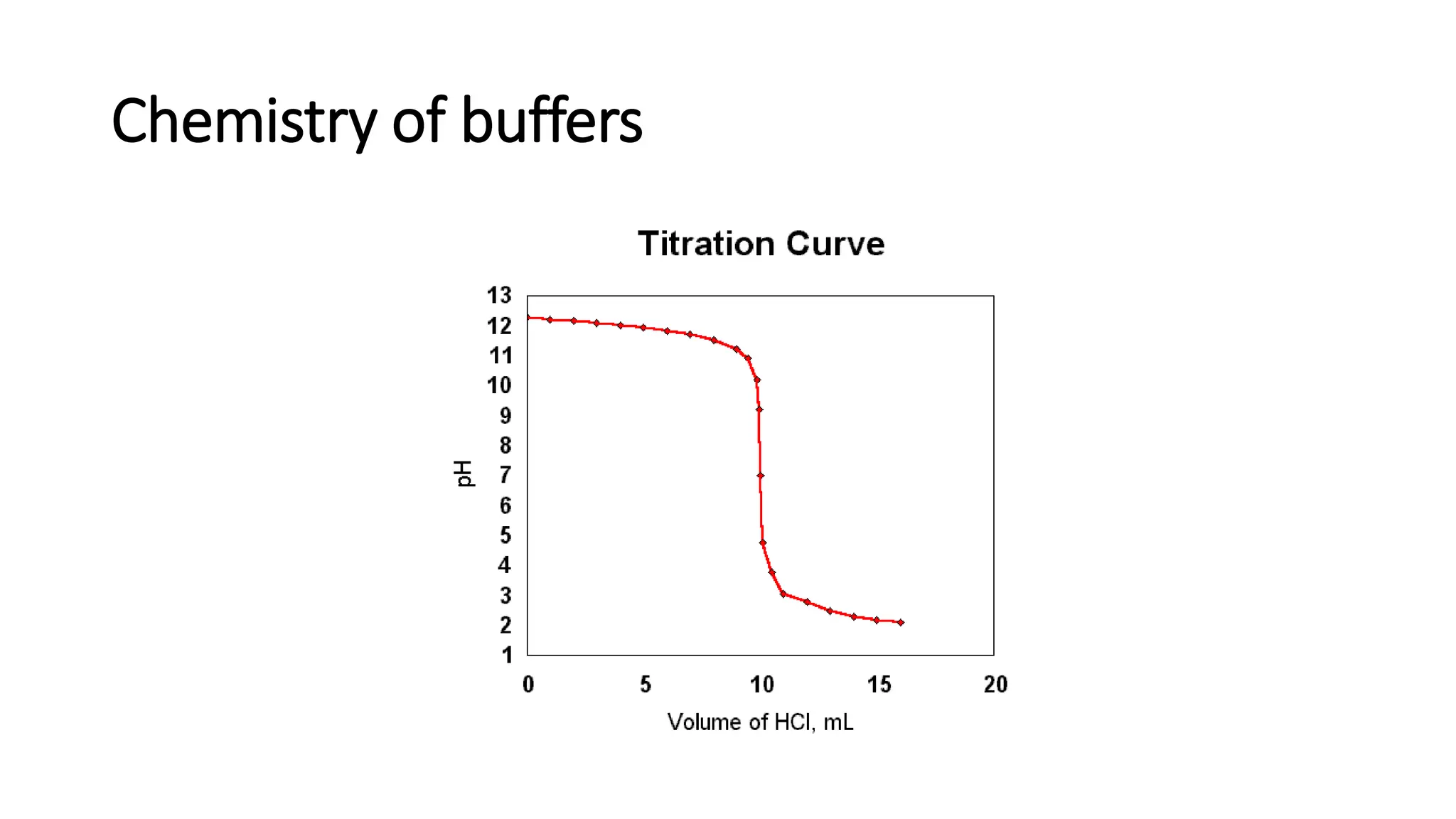
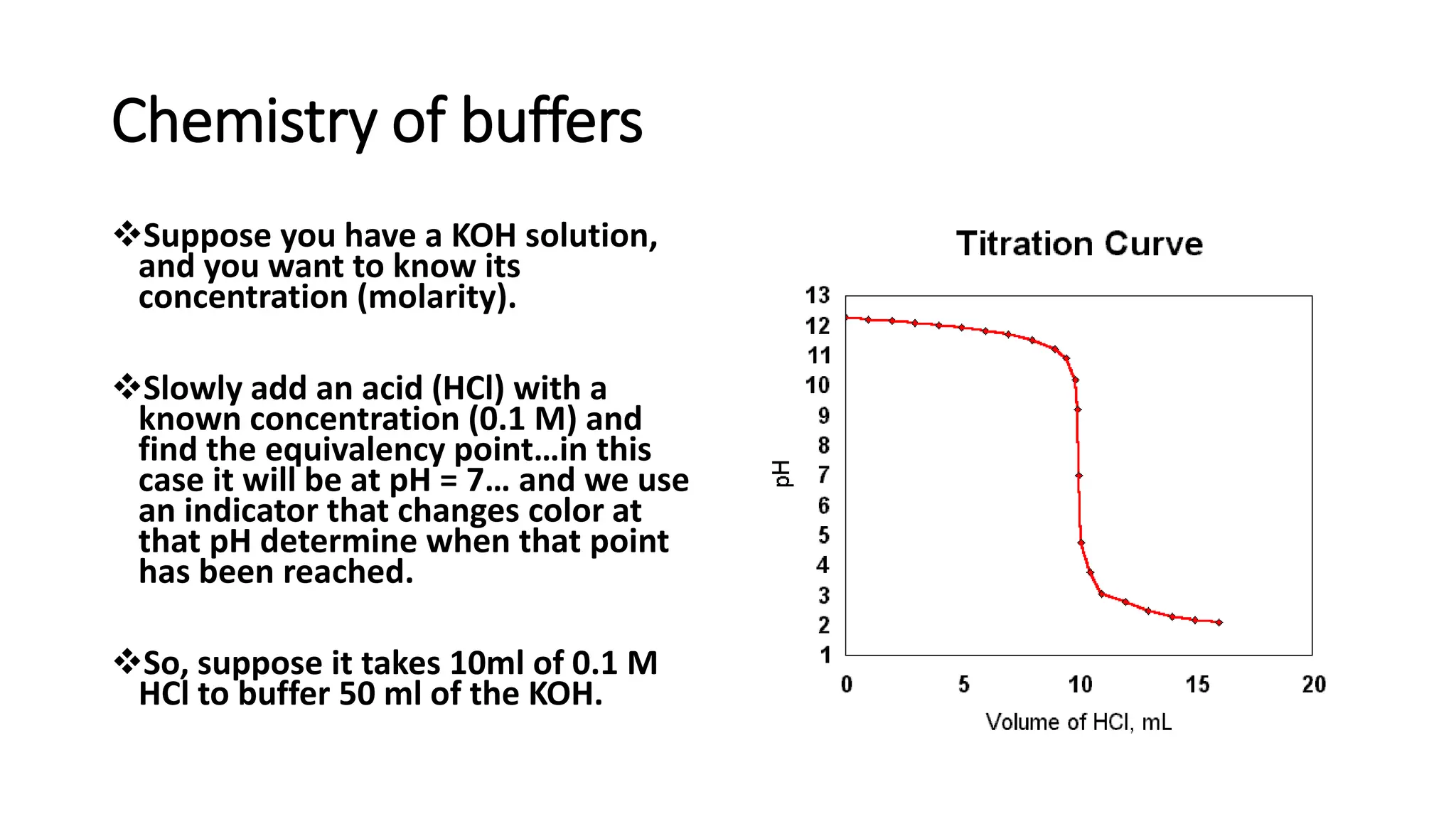
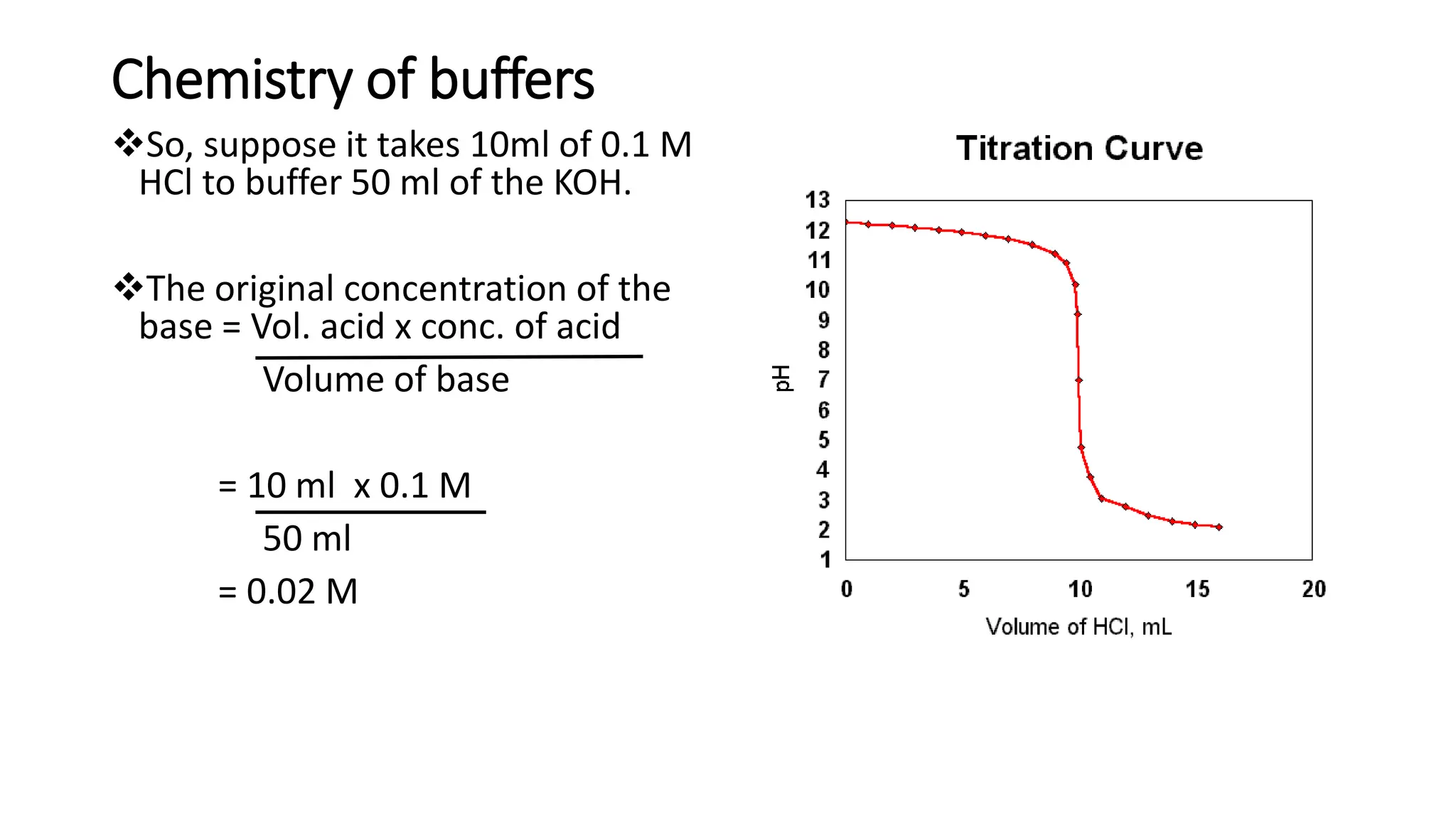
![pH range at Equivalence point: Titration curves
• pH range at equivalence point is obtained from the pH profile for a titration, a plot of
pH (of titrand solution) against volume of titrant added.
• pH shows a large increase (inflexion) at equivalence point.
• pH and pH range at equivalence point depend on strengths of acid and base used in
the titration.
• Indicator works best when its pKa is equal to pH at equivalence point; i.e. [HIn] = [In-]](https://image.slidesharecdn.com/buffers-240313060155-139f94fe/75/Buffers_Acidic-and-Basic-buffer-solutions-40-2048.jpg)