Here are potential responses to the study questions:
Define the following terms:
- Ionization: The process by which an atom or molecule acquires a negative or positive charge by gaining or losing electrons.
- Buffer capacity: The ability of a solution to resist changes in pH upon the addition of an acid or base. It depends on the buffer composition and concentration.
- In-vivo: Occurring or taking place inside a living organism.
Considering a practical process, illustrate the procedural significance of buffer systems in moderation of the reactions of a solution system:
Buffer systems are important in pharmaceutical formulations to maintain the pH within an optimal range for drug stability, solubility, and to minimize irritation upon administration.

![2
Ionization of Solutes:
Electrolytes
Are solutes which dissociate into ions if the dielectric constant of the
solvent is high enough to cause sufficient separation of the attractive
forces between the oppositely charged ions.
Ionization (dissociation) of electrolytes has several consequences e.g.
Hydrogen ion concentration and pH:
The dissociation of water can be represented by:
H2O H+ + OH-
In pure water the concentrations of H+ and OH- ions are equal and at
25°C both have the values of 1 x 10-7 mol/l.
Acid
a substance which donates a proton (or hydrogen ion)
the addition of an acid to water will increase hydrogen ion concentration
(more than 10-7 mol/l)
Base
a substance that accepts protons
the addition of a base will decrease the concentration of hydrogen ions.
[H3O+]](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-2-320.jpg)
![3
The hydrogen ion concentration range is from 1 mol/l for
a strong acid down to 1 x 10-14 mol/l for a strong base.
To avoid the use of such low values pH has been introduced
as a more convenient measure of hydrogen ion concentration.
pH is defined as the negative logarithm of the hydrogen ion
concentration [H+]
pH = -log10 [H+]
pH of a neutral solution like pure water is 7, why?
because the conc. of H +ions (and OH -) ions = 1 x 10-7 mol/l
pHs of acidic solutions will be less than 7
pHs of alkaline solutions will be greater than 7
pH has several important applications in pharmaceutical practice.
- Affect the solubilities of drugs that are weak acids or bases
- Affect the stabilities of many drugs
- Affect the ease of absorption of drugs from the GIT](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-3-320.jpg)
![4
Dissociation (or ionization) constants and pKa:
In solutions of weak acids or weak bases equilibria exist between
undissociated molecules and their ions.
For a weakly acidic drug HA: The ionization constant
(dissociation constant) Ka of a weak acid can be obtained by
applying the Law of Mass Action: HA H+ + A-
[H+] [A-] [HA]
Ka= pKa = pH + log
[HA] [A-]
pKa = the negative logarithm of Ka
Henderson-Hasselbalch equation:
A general equation that is applicable to any acidic drug with one
ionizable gp : Cu = conc. of the unionized Ci = conc. of the ionized species
Cu
pKa = pH + log
Ci](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-4-320.jpg)
![5
The ionization constant (dissociation constant) Ka of a protonated
weak base is given by B + H+ BH+
[H+] [B]
Ka=
[BH+]
Taking the negative log of this equation:
[BH+]
pKa = pH + log
[B]
Henderson-Hasselbalch equation:
A general equation that is applicable to any weak basic drug with
one ionizable group where:
Ci = conc. of protonated ; Cu = conc. of the unionized species
Ci
pKa = pH + log
Cu](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-5-320.jpg)
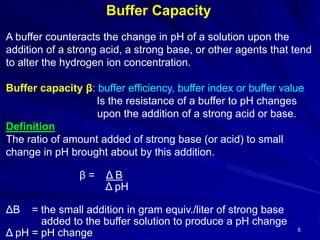
![7
The buffer capacity of the solution has a value of 1:
of strong base (or acid) togram equiv.1when the addition of
pH unit.1of the buffer solution results in a change ofliter1
Acetate bufferExample:
acetic acid & sodium acetate
0.1 mole each in 1 liter of solution.
a) 0.01 mole portions of NaOH is added
HAc + NaOH NaAc + H2O
(0.1 – 0.01) (0.01) (0.1 + 0.01)
b) The conc. of Na acetate (the [salt] in buffer equation) by 0.01 mol/l
& the conc. of acetic acid [acid] by 0.01 mol/l
because each addition of base converts 0.01 mole of acetic acid into
0.01 mole of sodium acetate according to the reaction.](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-7-320.jpg)
![8
Before the addition of the first portion of NaOH,
the pH of the buffer solution is:
pKa = pH + log pH = pKa - log
pH = pKa + log
pH = 4.76 + log (0.1) = 4.76
(0.1)
pH = pKa
The changes in concentration of the salt and the acid by the
addition of a base are represented by
pH = pKa + log pH = 4.76 + log[salt ] + [base]
[acid] - [base]
Cu [acid]
Ci [salt]
[acid]
[salt]
[salt ]
[acid]
(0.1) + 0.01
(0.1) – 0.01](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-8-320.jpg)
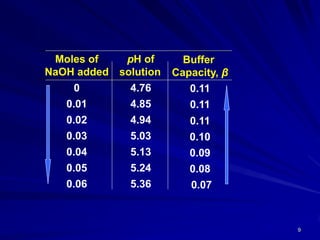
![10
The buffer capacity is not a fixed value for a given buffer
system, but depends on the amount of base added.
With the addition of more NaOH, the buffer capacity
decreases rapidly, and, when sufficient base has been
added the acid convert completely into sodium ions and
acetate ions
The buffer has it’s greatest capacity before any base is
added where [salt] / [acid] = 1, and according to equation,
pH = pKa.
The buffer capacity is influenced by an increase in the total
conc. of the buffer constituents since a greater conc. of salt
and acid provides a greater alkaline and acid reserve.](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-10-320.jpg)
![11
formore exact equationVan Slyke developed a
calculation of buffer capacity β
]+O3Ka [HC3.2=β
[H3O+])2+Ka)
C = The total buffer concentration (the sum of the molar
concentrations of the acid and the salt).
Ka = dissociation constant
H3O+ = hydrogen ion concentration
The equation permits the calculation of the buffer capacity
at any hydrogen ion concentration, i.e. when no acid or base
has been added to the buffer
[H+]](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-11-320.jpg)
![12
Example:
If hydrogen ion concentration is 1.75 x 10-5, pH = 4.76
what is the capacity of the buffer containing 0.10 mole of
each of acetic acid and sodium acetate per liter of solution ?
The total concentration , C = [acid] + [salt], is 0.20 mol/l and
the dissociation constant Ka is 1.75 x 10-5
]+O3Ka [HC3.2=β
(Ka + [H3O+])2
115.0=)5-10X75.1) x (5-10x75.1x (20.0x3.2β =
[(1.75x10-5) +(1.75 X 10-5)]2](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-12-320.jpg)





![18
The following steps should be used in preparing buffer systems
a. Select a weak acid having a pKa approximately equal to
the pH wanted to insure maximum buffer capacity.
b. From the buffer equation, calculate the ratio of salt and weak
acid required to obtain the desired pH.
log Cu = pKa - pH
Ci
c. Consider the individual concentrations of the buffer salt
and acid needed to obtain a suitable buffer capacity.
β = 2.3 C Ka [H3O+]
)Ka + [H3O+])2
A concentration of 0.05 to 0.5 molar is sufficient and
a buffer capacity of 0.01 to 0.1 is sufficient.
d. Finally, determine the pH and buffer capacity of the completed
buffered solution using a pH meter.](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-18-320.jpg)








![27
Study Questions
Define the following terms:
[Ionization, buffer capacity, in-vivo, etc]
Respond to the following questions:
Considering a practical process, illustrate the procedural significance of buffer systems in moderation of the
reactions of a solution system
What steps should be adopted to prepare a buffer system
Group work discussional questions:
Discuss the variations in a solution that may constitute the buffering effects of such
pharmaceutical solutions
What are the main key points to consider when a buffer system is being pharmaceutically
processed](https://image.slidesharecdn.com/4-buffersinpharmacy-180825211105/85/Buffers-in-Pharmacy-27-320.jpg)