1. Buffers resist changes in pH upon addition of acids or bases through neutralization reactions. Buffer capacity is a measure of this resistance to pH change and depends on the relative concentrations of weak acid/base and their conjugate salt in solution.
2. The maximum buffer capacity occurs when the pH equals the pKa of the buffering species. Common pharmaceutical buffers use weak acids like acetic acid and their conjugate bases to maintain pH in a specified range.
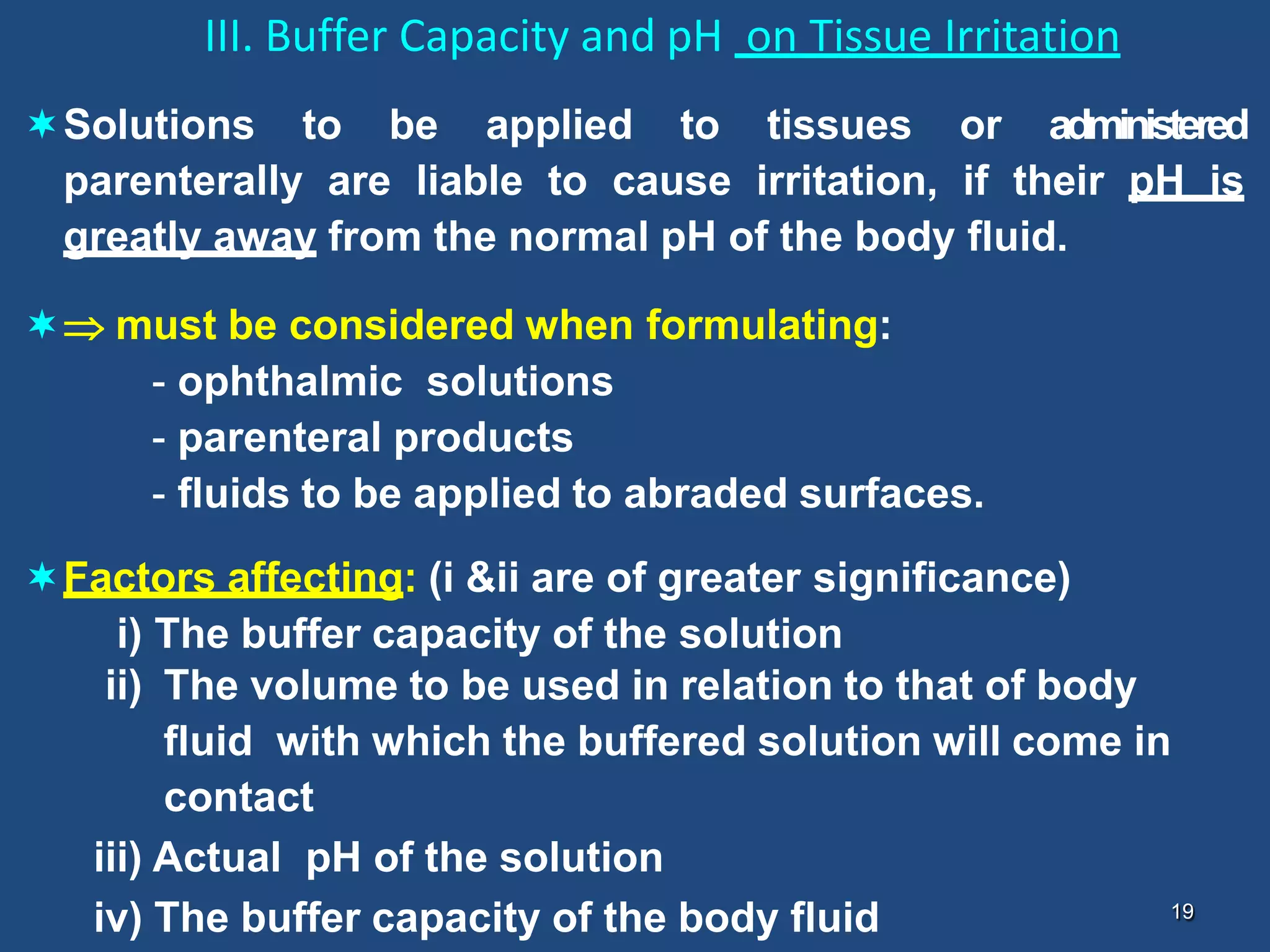
3. Blood and tears are important biological buffer systems that maintain near-neutral pH. Pharmaceutical formulations also use buffers to control pH and prevent irritation when administered. Proper buffer selection and concentrations are important to achieve sufficient capacity while avoiding toxicity.

![7
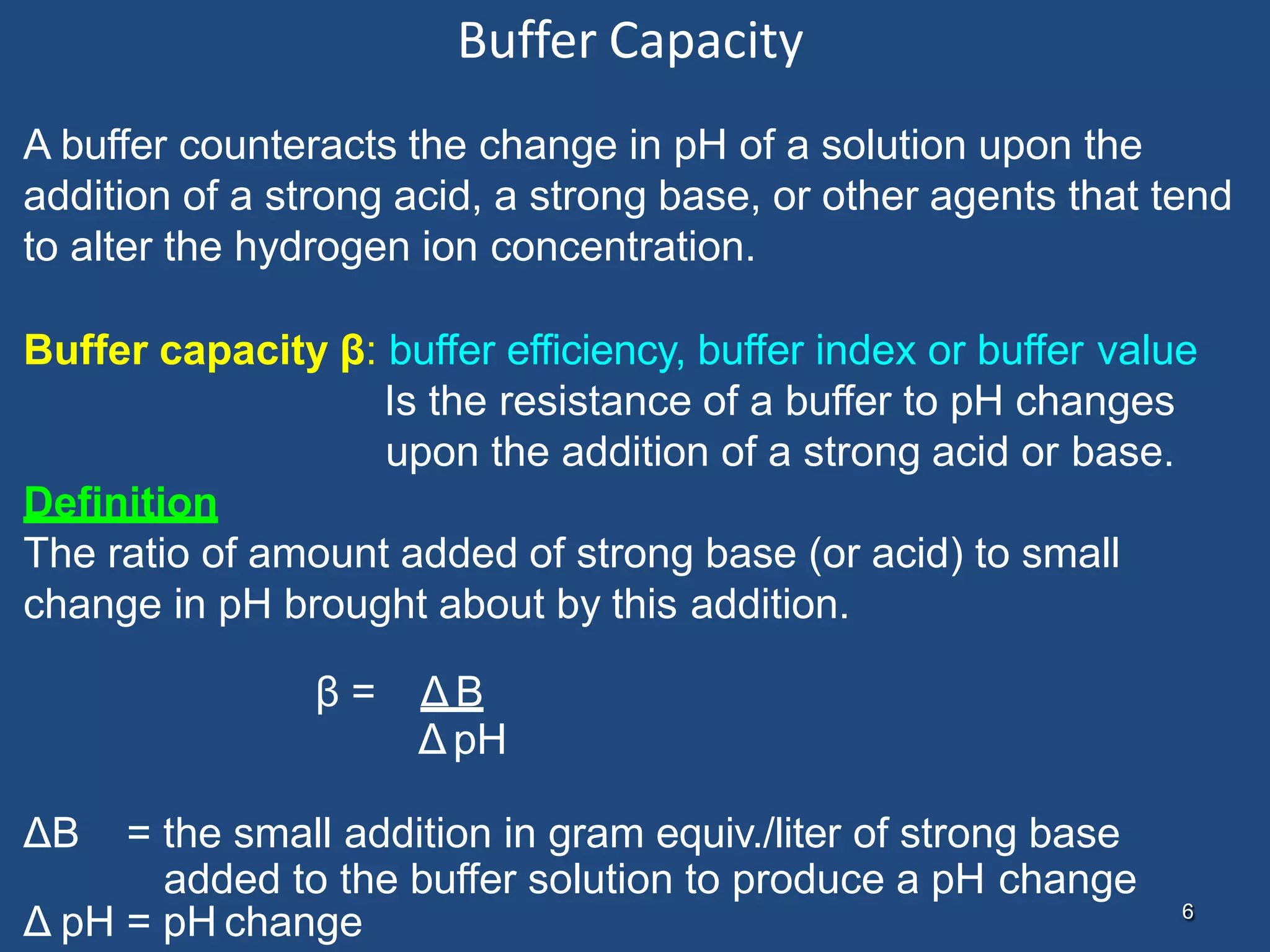
The buffer capacity of the solution has a value of 1:
when the addition of 1 gram equiv. of strong base (or acid) to 1 liter of
the buffer solution results in a change of 1 pH unit.
Example: Acetate buffer
acetic acid & sodium acetate
0.1 mole each in 1 liter of solution.
a) 0.01 mole portions of NaOH is added
HAc +
(0.1 – 0.01)
NaOH
(0.01)
NaAc + H2O
(0.1 + 0.01)
b) The conc. of Na acetate (the [salt] in buffer equation) by 0.01 mol/l
& the conc. of acetic acid [acid] by 0.01 mol/l
because each addition of base converts 0.01 mole of acetic acid into
0.01 mole of sodium acetate according to the reaction.](https://image.slidesharecdn.com/buffercapacity-210130074316/75/Buffer-capacity-3-2048.jpg)
![Before the addition of the first portion of NaOH,
the pH of the buffer solution is:
pH = pKa + log [salt ] + [base]
[acid] - [base]
pKa = pH + log Cu [acid]
Ci [salt]
pH = pKa - log [acid]
[salt]
pH = pKa + log [salt ]
[acid]
pH = 4.76 + log (0.1) = 4.76
(0.1)
pH = pKa
The changes in concentration of the salt and the acid by the
addition of a base are represented by
pH = 4.76 + log (0.1) + 0.01
(0.1) – 0.018](https://image.slidesharecdn.com/buffercapacity-210130074316/75/Buffer-capacity-4-2048.jpg)

![10
The buffer capacity is not a fixed value for a given buffer
system, but depends on the amount of base added.
With the addition of more NaOH, the
decreases rapidly, and, when sufficient
buffer capacity
base has been
added the acid convert completely into sodium ions and
acetate ions
The buffer has it’s greatest capacity before any base is
added where [salt] / [acid] = 1, and according to equation,
pH = pKa.
The buffer capacity is influenced by an increase in the total
conc. of the buffer constituents since a greater conc. of salt
and acid provides a greater alkaline and acid reserve.](https://image.slidesharecdn.com/buffercapacity-210130074316/75/Buffer-capacity-6-2048.jpg)
![11
Van Slyke developed a more exact equation for
calculation of buffer capacity β
β = 2.3 C Ka [H3O+]
(Ka +[H3O+])2
C = The total buffer concentration (the sum of the molar
concentrations of the acid and the salt).
Ka = dissociation constant
H3O+ = hydrogen ionconcentration
The equation permits the calculation of the buffer capacity at any
hydrogen ion concentration, i.e. when no acid or base has been
added to the buffer
[H+]](https://image.slidesharecdn.com/buffercapacity-210130074316/75/Buffer-capacity-7-2048.jpg)
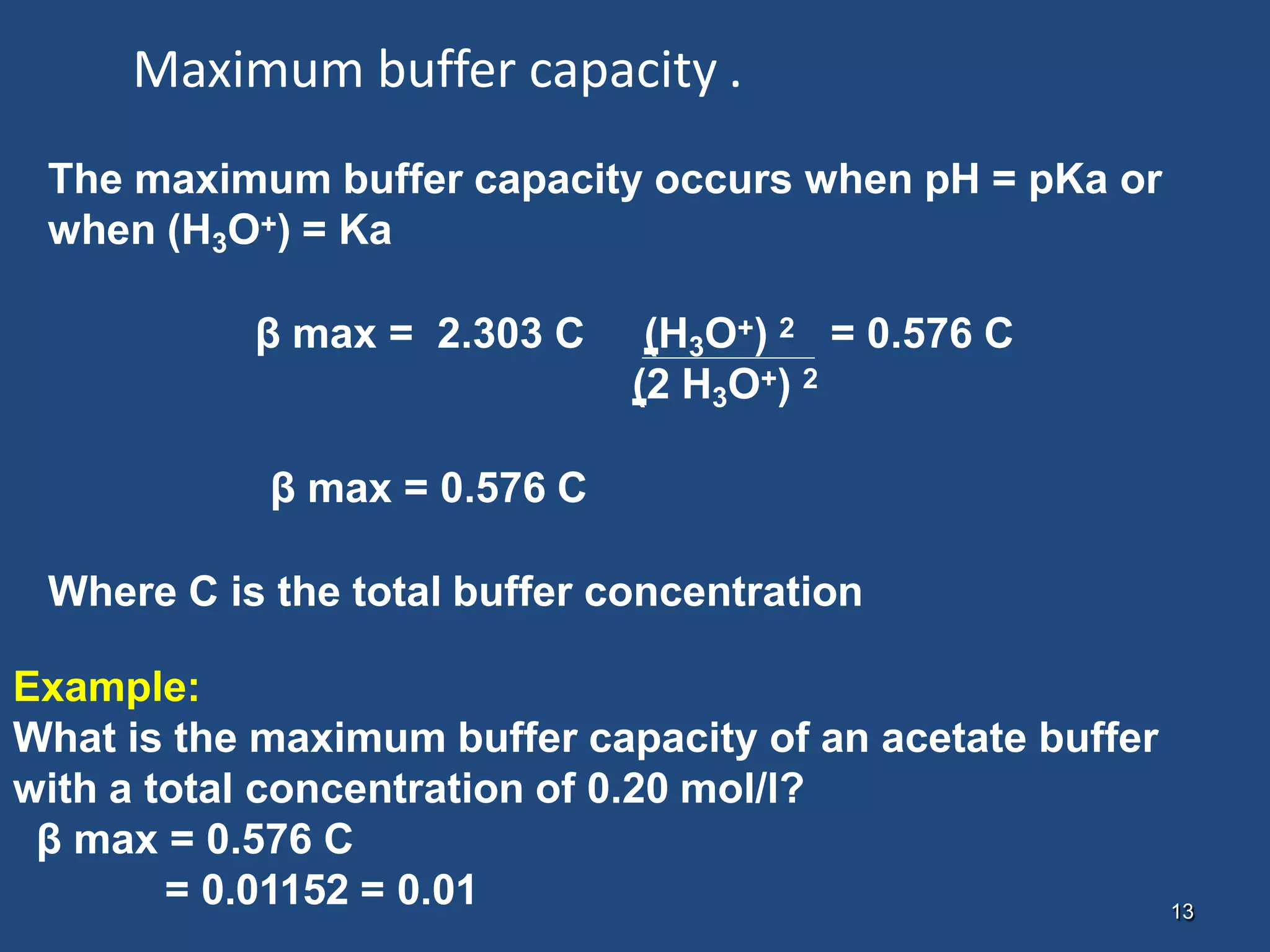
![12
Example:
If hydrogen ion concentration is 1.75 x 10-5, pH = 4.76
what is the capacity of the buffer containing 0.10 mole of
each of acetic acid and sodium acetate per liter of solution ?
The total concentration , C = [acid] + [salt], is 0.20 mol/l and
the dissociation constant Ka is 1.75 x 10-5
β = 2.3 C Ka [H3O+]
(Ka + [H3O+])2
β = 2.3 x 0.20 x (1.75x10-5) x (1.75 X 10-5) = 0.115
[(1.75x10-5) +(1.75 X 10-5)]2](https://image.slidesharecdn.com/buffercapacity-210130074316/75/Buffer-capacity-8-2048.jpg)



![18
The following steps should be used in preparing buffer systems
a. Select a weak acid having a pKa approximately equal to the pH
wanted to insure maximum buffer capacity.
b. From the buffer equation, calculate the ratio of salt and weak
acid required to obtain the desired pH.
log Cu = pKa - pH
Ci
c. Consider the individual concentrations of the buffer salt
and acid needed to obtain a suitable buffer capacity.
β = 2.3 C Ka [H3O+]
(Ka + [H3O+])2
A concentration of 0.05 to 0.5 molar is sufficient and
a buffer capacity of 0.01 to 0.1 is sufficient.
d. Finally, determine the pH and buffer capacity of the completed
buffered solution using a pH meter.](https://image.slidesharecdn.com/buffercapacity-210130074316/75/Buffer-capacity-14-2048.jpg)