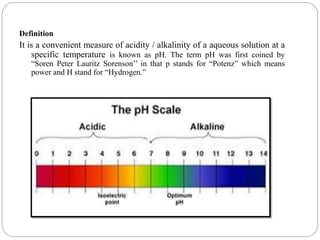
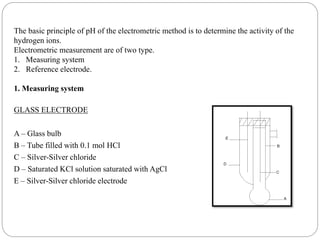
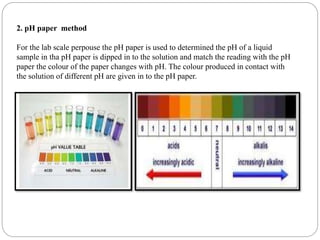
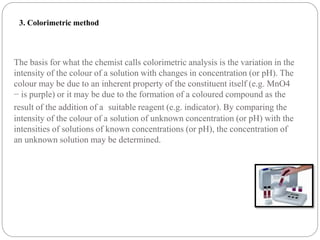
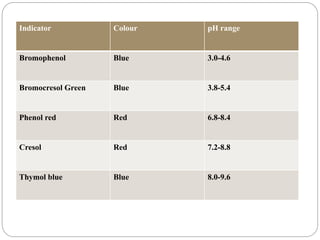
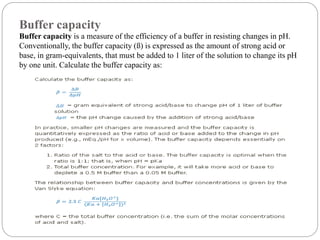
This document discusses pH buffers and isotonic solutions. It defines pH as a measure of acidity or alkalinity of an aqueous solution, outlines methods of pH measurement including electrometric, pH paper, and colorimetric methods, and discusses the importance and applications of buffers in biological and pharmaceutical systems like blood and tears which require precise pH regulation. It also defines isotonic solutions as those that do not cause cell contraction or swelling when injected and discusses related concepts like osmotic pressure, osmolality, and osmolarity.