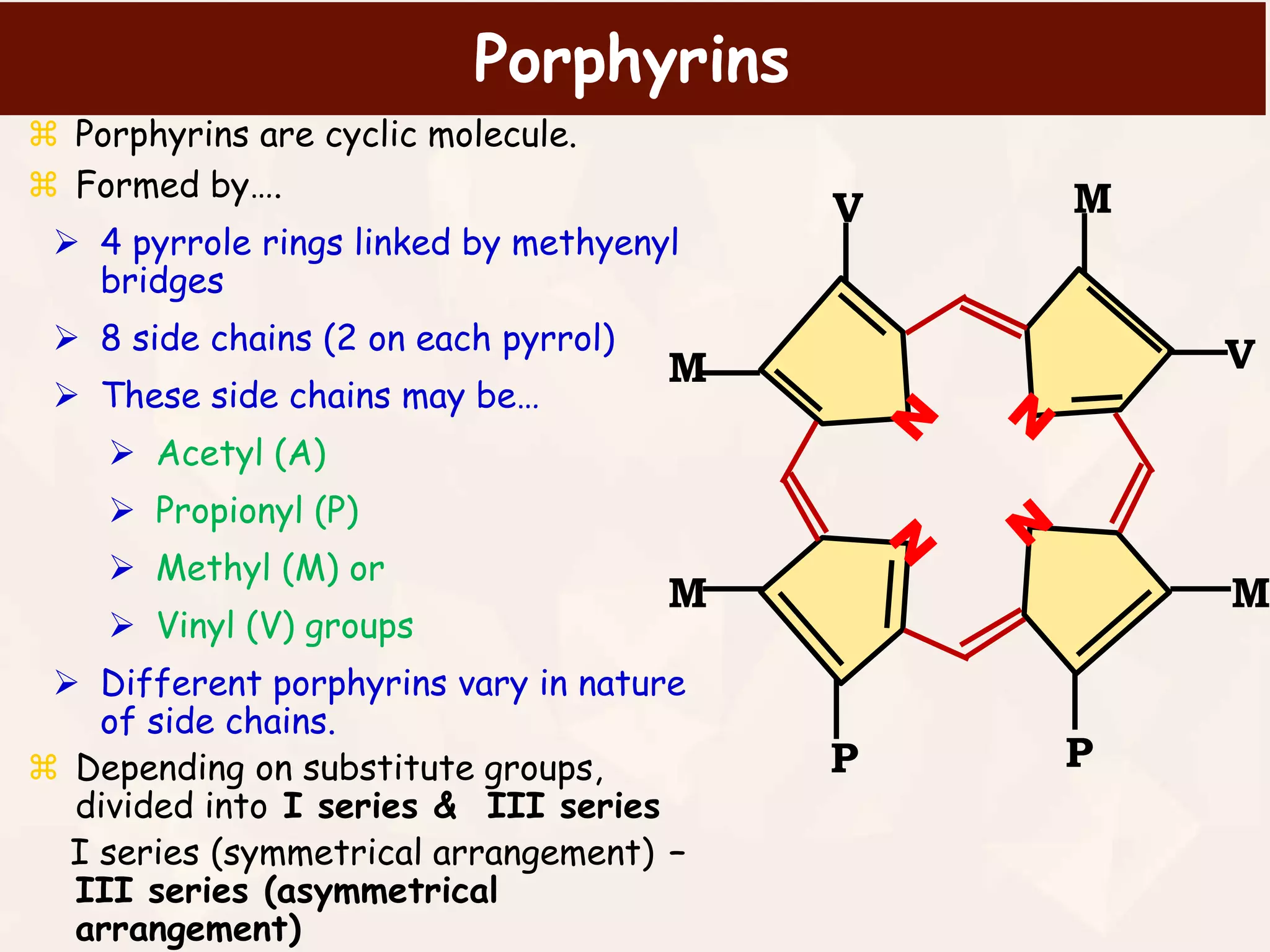
Hemoglobin is a protein in red blood cells that transports oxygen and carbon dioxide throughout the body. It is made up of four subunits, each containing a heme group with iron at its center. Heme biosynthesis is a multi-step pathway that takes place in the mitochondria and cytoplasm, starting from succinyl-CoA and glycine and resulting in protoporphyrin with iron inserted at the final step to form heme. Regulation of heme biosynthesis occurs through feedback inhibition of the rate-limiting enzyme ALA synthase by heme levels. Deficiencies in the heme biosynthesis pathway can cause various types of porphyrias, a group of rare genetic disorders characterized by neurological and skin abnormalities.