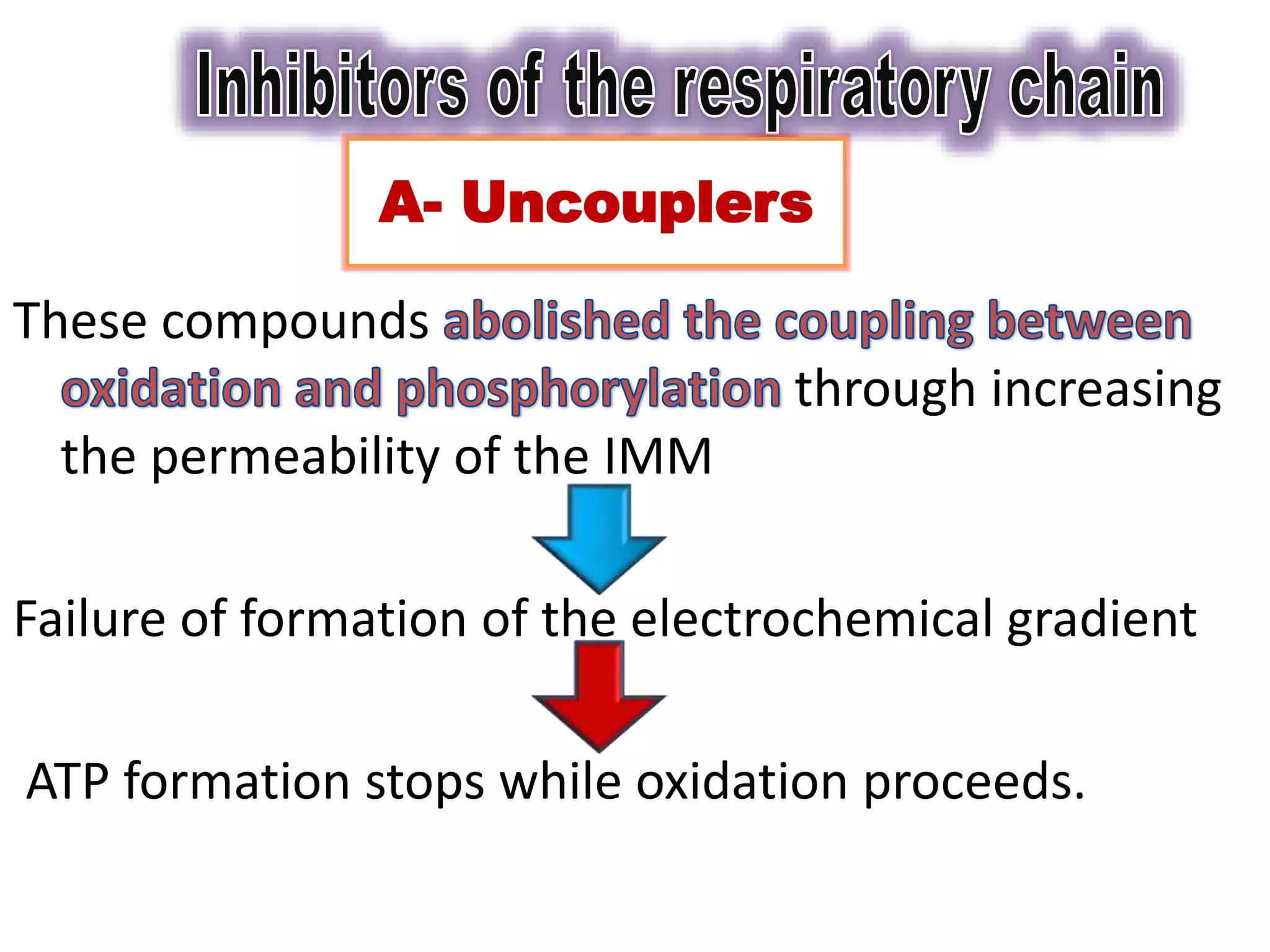
ATP moves from the mitochondrial matrix to the cytosol via the ATP-ADP translocase membrane transport protein. The translocase tightly couples the exchange of ADP for ATP as ATP exits. Uncouplers act to transport hydrogen ions across the inner mitochondrial membrane to the matrix without passing through ATP synthase. This short-circuits the proton gradient and results in energy being released as heat rather than being used to synthesize ATP. The malate-aspartate shuttle transfers reducing equivalents in the form of NADH from the cytosol into the mitochondrial matrix.