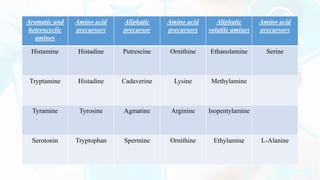
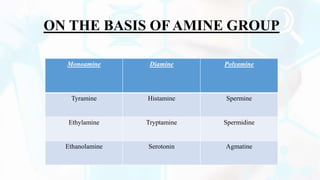
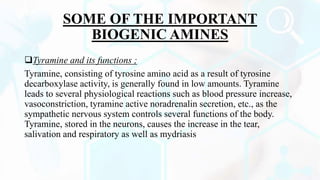
Deamination and decarboxylation are processes that break down amino acids. Deamination removes an amine group from an amino acid, releasing ammonia. There are two types of deamination - oxidative deamination uses oxidation to remove the amine group, while non-oxidative uses other reactions. Decarboxylation removes a carboxyl group from an amino acid, releasing carbon dioxide. Both processes help convert excess amino acids into usable byproducts that can be removed from the body.